Efficacy of weekly versus daily cholecalciferol for repleting serum vitamin D (25(OH)D) deficiency: A systematic review and meta-analysis of randomized controlled trials
- PMID: 39396907
- PMCID: PMC11617645
- DOI: 10.1111/bcpt.14092
Efficacy of weekly versus daily cholecalciferol for repleting serum vitamin D (25(OH)D) deficiency: A systematic review and meta-analysis of randomized controlled trials
Abstract
Background/rationale: Weekly cholecalciferol can replace daily supplementation to reduce pill burden in patients with complex medication regimens and hypovitaminosis D, but evidence supporting this switch is unclear.
Objective: We aimed to determine whether weekly cholecalciferol was superior to daily cholecalciferol to replete patients with hypovitaminosis D.
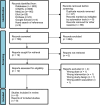
Methods: We conducted a systematic review of randomized controlled trials involving participants with baseline hypovitaminosis D (<30 ng/ml) comparing weekly versus daily cholecalciferol dosing and where serum cholecalciferol was measured within 120 days of starting treatment. We searched MEDLINE, CINAHL and EMBASE from inception to 7 May 2024. A random-effects meta-analysis evaluated the odds ratio for repletion of serum vitamin D levels.
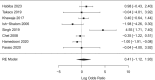
Findings: Eight trials involving 542 patients were included in the analysis. Weekly and daily cholecalciferol were not significantly different in correcting hypovitaminosis D (OR = 1.5, 95% CI = 0.3-6.9, p = 0.6, favouring weekly dosing, I2 = 85.3%). A sensitivity analysis excluding otherwise healthy patients had similar findings (OR = 0.8, 95% CI = 0.3-2.1, p = 0.6). Most studies were at risk of bias; the different doses being compared increased the heterogeneity.
Conclusions: Limited direct evidence supports a switch from daily to weekly cholecalciferol dosing; however, weekly supplementation was not demonstrably worse at repleting levels and decreased a patient's daily pill burden.
Keywords: cholecalciferol; deprescribing; dosing strategy; meta‐analysis; systematic review.
© 2024 The Author(s). Basic & Clinical Pharmacology & Toxicology published by John Wiley & Sons Ltd on behalf of Nordic Association for the Publication of BCPT (former Nordic Pharmacological Society).
Conflict of interest statement
None of the other authors have any relevant conflicts of interest to declare.
Figures
References
-
- Sluggett JK, Chen EYH, Ilomäki J et al. Reducing the burden of complex medication regimens: SImplification of medications prescribed to long‐tErm care residents (SIMPLER) cluster randomized controlled trial. J am Med Dir Assoc 2020/08/01/2020;21(8):1114‐1120.e4. doi: 10.1016/j.jamda.2020.02.003 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical