Efficacy and safety of venetoclax combination therapy for relapsed/refractory acute myeloid leukemia: a systematic review and meta-analysis
- PMID: 39396935
- PMCID: PMC11472599
- DOI: 10.1186/s12885-024-13000-3
Efficacy and safety of venetoclax combination therapy for relapsed/refractory acute myeloid leukemia: a systematic review and meta-analysis
Abstract
Background: As we delve into the intricate world of venetoclax combination therapy in relapsed or refractory acute myeloid leukemia (AML), our exploration not only aims to contribute to the current body of knowledge but also strives to inform future research directions, clinical decision-making, and the ongoing evolution of therapeutic strategies in the relentless pursuit of improved outcomes for patients facing this formidable hematologic malignancy.
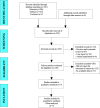
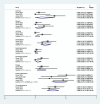
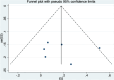
Methods: We systematically searched PubMed, Embase, and Cochrane databases from inception to November 2023 for English-language studies on venetoclax combination therapy in relapsed/refractory AML. We excluded duplicate published studies, incomplete studies, those with incomplete data, animal experiments, literature reviews, and systematic studies. Meta-analysis was performed using STATA 15.1.
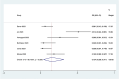
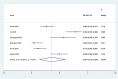
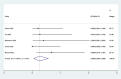
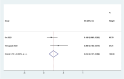
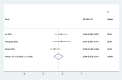
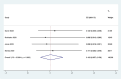
Results: Out of 58 identified articles, seven were included in the meta-analysis. The pooled complete remission (CR) rate was 15.4%, and the composite complete remission (CRc) rate was 35.7%. The partial remission (PR) rate was 2.6%, while the non-remission (NR) rate was 24.4%. The minimal residual disease status in CRc patients (MRD-CRc) rate was 39.4%, and the morphologic leukemia-free state (MLFS) rate was 10.3%. Incidence of adverse events included diarrhea (10.0%), nausea (4.3%), vomiting (2.6%), hypokalemia (16.4%), hypomagnesemia (0.8%), decreased appetite (4.2%), fatigue (9.1%), febrile neutropenia (39.6%), and thrombocytopenia (28.4%). Subgroup analysis based on combined drugs revealed varying CR and CRc rates. the combination of venetoclax and azacitidine + demonstrates superior outcomes, displaying the highest rates of CR at 31.3% and CRc at 62.7%. In contrast, venetoclax and idasanutlin exhibits a moderate CR rate of 6.1% and a CRc rate of 26.5%, while venetoclax and mivebresib shows the lowest CR rate at 3.3% and a moderate CRc rate of 8.0%.
Conclusion: In conclusion, while venetoclax combination therapies, particularly with azacitidine + , show promise in achieving favorable treatment responses in relapsed/refractory AML patients, a comprehensive evaluation of safety profiles is essential. Nevertheless, it is essential to underscore the markedly increased incidence rates of febrile neutropenia and thrombocytopenia observed among adverse events.
Keywords: Acute myeloid leukemia; Efficacy and safety; Meta-analysis; Relapsed/refractory; Systematic review; Venetoclax.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

