Neuronal activity in the ventral tegmental area during goal-directed navigation recorded by low-curvature microelectrode arrays
- PMID: 39396959
- PMCID: PMC11471829
- DOI: 10.1038/s41378-024-00778-2
Neuronal activity in the ventral tegmental area during goal-directed navigation recorded by low-curvature microelectrode arrays
Abstract
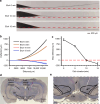
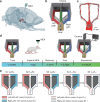
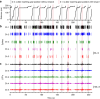
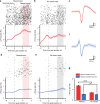
Navigating toward destinations with rewards is a common behavior among animals. The ventral tegmental area (VTA) has been shown to be responsible for reward coding and reward cue learning, and its response to other variables, such as kinematics, has also been increasingly studied. These findings suggest a potential relationship between animal navigation behavior and VTA activity. However, the deep location and small volume of the VTA pose significant challenges to the precision of electrode implantation, increasing the uncertainty of measurement results during animal navigation and thus limiting research on the role of the VTA in goal-directed navigation. To address this gap, we innovatively designed and fabricated low-curvature microelectrode arrays (MEAs) via a novel backside dry etching technique to release residual stress. Histological verification confirmed that low-curvature MEAs indeed improved electrode implantation precision. These low-curvature MEAs were subsequently implanted into the VTA of the rats to observe their electrophysiological activity in a freely chosen modified T-maze. The results of the behavioral experiments revealed that the rats could quickly learn the reward probability corresponding to the left and right paths and that VTA neurons were deeply involved in goal-directed navigation. Compared with those in no-reward trials, VTA neurons in reward trials presented a significantly greater firing rate and larger local field potential (LFP) amplitude during the reward-consuming period. Notably, we discovered place fields mapped by VTA neurons, which disappeared or were reconstructed with changes in the path-outcome relationship. These results provide new insights into the VTA and its role in goal-directed navigation. Our designed and fabricated low-curvature microelectrode arrays can serve as a new device for precise deep brain implantation in the future.
© 2024. The Author(s).
Conflict of interest statement
Xinxia Cai is an editor for the journal, no other author has reported any competing interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous
