Robotic microinjection enables large-scale transgenic studies of Caenorhabditis elegans
- PMID: 39397017
- PMCID: PMC11471809
- DOI: 10.1038/s41467-024-53108-5
Robotic microinjection enables large-scale transgenic studies of Caenorhabditis elegans
Abstract
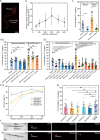
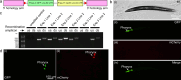
The nematode Caenorhabditis elegans is widely employed as a model organism to study basic biological mechanisms. However, transgenic C. elegans are generated by manual injection, which remains low-throughput and labor-intensive, limiting the scope of approaches benefitting from large-scale transgenesis. Here, we report a robotic microinjection system, integrating a microfluidic device capable of reliable worm immobilization, transfer, and rotation, for high-speed injection of C. elegans. The robotic system provides an injection speed 2-3 times faster than that of experts with 7-22 years of experience while maintaining comparable injection quality and only limited trials needed by users to become proficient. We further employ our system in a large-scale reverse genetic screen using multiplexed alternative splicing reporters, and find that the TDP-1 RNA-binding protein regulates alternative splicing of zoo-1 mRNA, which encodes variants of the zonula occludens tight junction proteins. With its high speed, high accuracy, and high efficiency in worm injection, this robotic system shows great potential for high-throughput transgenic studies of C. elegans.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- White, J. G., Southgate, E., Thomson, J. N. & Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. L. B Biol. Sci.314, 1–340 (1986). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RGPIN-2017-06374/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- RGPIN-2022-05039/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- JELF-38428/Canada Foundation for Innovation (Fondation canadienne pour l'innovation)
- 389077/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
LinkOut - more resources
Full Text Sources
Research Materials

