Aptamer-drug conjugates-loaded bacteria for pancreatic cancer synergistic therapy
- PMID: 39397032
- PMCID: PMC11471780
- DOI: 10.1038/s41392-024-01973-3
Aptamer-drug conjugates-loaded bacteria for pancreatic cancer synergistic therapy
Abstract
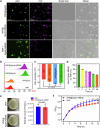
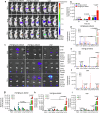
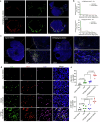
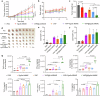
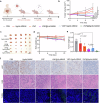
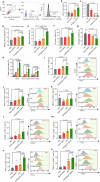
Pancreatic cancer is one of the most malignant tumors with the highest mortality rates, and it currently lacks effective drugs. Aptamer-drug conjugates (ApDC), as a form of nucleic acid drug, show great potential in cancer therapy. However, the instability of nucleic acid-based drugs in vivo and the avascularity of pancreatic cancer with dense stroma have limited their application. Fortunately, VNP20009, a genetically modified strain of Salmonella typhimurium, which has a preference for anaerobic environments, but is toxic and lacks specificity, can potentially serve as a delivery vehicle for ApDC. Here, we propose a synergistic therapy approach that combines the penetrative capability of bacteria with the targeting and toxic effects of ApDC by conjugating ApDC to VNP20009 through straightforward, one-step click chemistry. With this strategy, bacteria specifically target pancreatic cancer through anaerobic chemotaxis and subsequently adhere to tumor cells driven by the aptamer's specific binding. Results indicate that this method prolongs the serum stability of ApDC up to 48 h and resulted in increased drug concentration at tumor sites compared to the free drugs group. Moreover, the aptamer's targeted binding to cancer cells tripled bacterial colonization at the tumor site, leading to increased death of tumor cells and T cell infiltration. Notably, by integrating chemotherapy and immunotherapy, the effectiveness of the treatment is significantly enhanced, showing consistent results across various animal models. Overall, this strategy takes advantage of bacteria and ApDC and thus presents an effective synergistic strategy for pancreatic cancer treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

