ARL13B controls male reproductive tract physiology through primary and Motile Cilia
- PMID: 39397107
- PMCID: PMC11471856
- DOI: 10.1038/s42003-024-07030-7
ARL13B controls male reproductive tract physiology through primary and Motile Cilia
Abstract
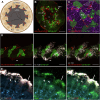
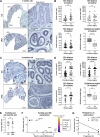
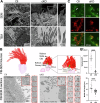
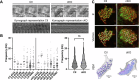
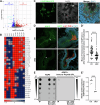
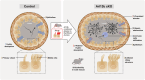
ARL13B is a small regulatory GTPase that controls ciliary membrane composition in both motile cilia and non-motile primary cilia. In this study, we investigated the role of ARL13B in the efferent ductules, tubules of the male reproductive tract essential to male fertility in which primary and motile cilia co-exist. We used a genetically engineered mouse model to delete Arl13b in efferent ductule epithelial cells, resulting in compromised primary and motile cilia architecture and functions. This deletion led to disturbances in reabsorptive/secretory processes and triggered an inflammatory response. The observed male reproductive phenotype showed significant variability linked to partial infertility, highlighting the importance of ARL13B in maintaining a proper physiological balance in these small ducts. These results emphasize the dual role of both motile and primary cilia functions in regulating efferent duct homeostasis, offering deeper insights into how cilia related diseases affect the male reproductive system.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Hess, R. A. The efferent ductules:Stucture and functions. The Epidid (2002).
MeSH terms
Substances
Grants and funding
- 75N91019D00024/CA/NCI NIH HHS/United States
- R01 HD104672/HD/NICHD NIH HHS/United States
- IC119871/Gouvernement du Canada | Instituts de Recherche en Santé du Canada | CIHR Skin Research Training Centre (Skin Research Training Centre)
- HD104672-01/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

