Deciphering the intracellular forces shaping mitochondrial motion
- PMID: 39397143
- PMCID: PMC11471753
- DOI: 10.1038/s41598-024-74734-5
Deciphering the intracellular forces shaping mitochondrial motion
Abstract
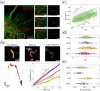
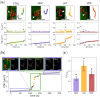
We propose a novel quantitative method to explore the forces affecting mitochondria within living cells in an almost non-invasive fashion. This new tool enables the detection of localized mechanical impulses on these organelles that occur amidst the stationary fluctuations caused by the thermal jittering in the cytoplasm. Recent experimental evidence shows that the action of mechanical forces has important effects on the dynamics, morphology and distribution of mitochondria in cells. In particular, their crosstalk with the cytoskeleton has been found to alter these organelles function; however, the mechanisms underlying this phenomenon are largely unknown. Our results highlight the different functions that cytoskeletal networks play in shaping mitochondrial dynamics. This work presents a novel technique to extend our knowledge of how the impact of mechanical cues can be quantified at the single organelle level. Moreover, this approach can be expanded to the study of other organelles or biopolymers.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Mitochondrial cellular organization and shape fluctuations are differentially modulated by cytoskeletal networks.Sci Rep. 2023 Mar 11;13(1):4065. doi: 10.1038/s41598-023-31121-w. Sci Rep. 2023. PMID: 36906690 Free PMC article.
-
Morphological fluctuations of individual mitochondria in living cells.J Phys Condens Matter. 2021 Dec 15;34(9). doi: 10.1088/1361-648X/ac3e9c. J Phys Condens Matter. 2021. PMID: 34847540
-
Regulation of mitochondrial structure by the actin cytoskeleton.Cytoskeleton (Hoboken). 2024 Apr-May;81(4-5):206-214. doi: 10.1002/cm.21804. Epub 2023 Nov 6. Cytoskeleton (Hoboken). 2024. PMID: 37929797 Review.
-
Mitochondria: A worthwhile object for ultrastructural qualitative characterization and quantification of cells at physiological and pathophysiological states using conventional transmission electron microscopy.Acta Histochem. 2020 Dec;122(8):151646. doi: 10.1016/j.acthis.2020.151646. Epub 2020 Oct 28. Acta Histochem. 2020. PMID: 33128989 Review.
-
Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function.Annu Rev Physiol. 2016;78:505-31. doi: 10.1146/annurev-physiol-021115-105011. Epub 2015 Nov 19. Annu Rev Physiol. 2016. PMID: 26667075 Review.
Cited by
-
Retrograde rearrangement of mitochondria correlates with nuclear deformation and genotoxic damage.iScience. 2025 Jun 19;28(8):112955. doi: 10.1016/j.isci.2025.112955. eCollection 2025 Aug 15. iScience. 2025. PMID: 40727930 Free PMC article.
References
-
- Su, É., Villard, C. & Manneville, J.-B. Mitochondria: At the crossroads between mechanobiology and cell metabolism. Biol. Cell115(9), e2300010 (2023). - PubMed
-
- Phuyal, S., Romani, P., Dupont, S. & Farhan, H. Mechanobiology of organelles: Illuminating their roles in mechanosensing and mechanotransduction. Trends Cell Biol.33(12), 1049–1061 (2023). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

