Involvement of BK Channels and Ryanodine Receptors in Salicylate-induced Tinnitus
- PMID: 39397241
- PMCID: PMC11880135
- DOI: 10.1007/s12035-024-04533-6
Involvement of BK Channels and Ryanodine Receptors in Salicylate-induced Tinnitus
Abstract
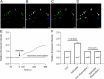
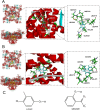
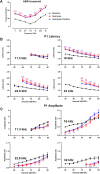
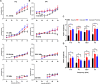
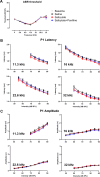
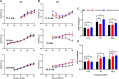
Neural hyperexcitability of the central auditory system is a key pathological characteristic of tinnitus, but its underlying molecular mechanisms remain elusive. The large-conductance Ca2+-activated K+ channel (BK) plays a crucial role in down- or upregulating neuronal activity. This study aims to investigate the role of BK channels in mediating tinnitus-associated neural hyperexcitability and elucidate the mechanisms behind it. Immunofluorescent staining revealed extensive expression of the BK channels on neurons within the central auditory system of rats. After long-term systemic administration of salicylate, a stable tinnitus inducer, we observed a significant change in the expression levels of BKα and β4 subunits in the rat central auditory system. In addition, salicylate was found to enhance the outward potassium currents mediated by the BK channel when exogenously expressed in HEK293 cells. Interestingly, this effect could be blocked by ryanodine, a potent inhibitor of ryanodine receptors (RyRs). Molecular docking identified Gln4020 within the central domain of RyR as a key residue in RyR-salicylate interactions. The results indicated that salicylate might directly activate RyRs leading to Ca2+ release from endoplasmic reticulum, and increased BK currents subsequently. Systemic treatment with paxilline, a potent blocker of BK channel, selectively reversed the increased P4/P1 amplitude ratios in the frequency region of tinnitus perception induced by single-dose salicylate administration. These results suggest that BK channels and ryanodine receptors may play a selective role in salicylate-induced tinnitus.
Keywords: BK channel; Hyperexcitability; Ryanodine receptor; Salicylate; Tinnitus.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: All procedures involving animals were carried out in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals, and were approved by the Ethics Committee of Hebei University, China. Consent to Participate: Not applicable. Consent for Publication: All authors have read and approved the final manuscript for publication. Competing Interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

