Targeting NAD+ Metabolism Vulnerability in FH-Deficient Hereditary Leiomyomatosis and Renal Cell Carcinoma with the Novel NAMPT Inhibitor OT-82
- PMID: 39397296
- PMCID: PMC12278184
- DOI: 10.1158/1535-7163.MCT-24-0225
Targeting NAD+ Metabolism Vulnerability in FH-Deficient Hereditary Leiomyomatosis and Renal Cell Carcinoma with the Novel NAMPT Inhibitor OT-82
Abstract
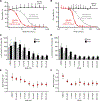
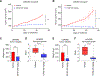
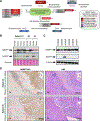
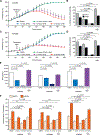
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is an inherited cancer syndrome caused by germline pathogenic variants in the fumarate hydratase (FH) gene. Affected individuals are at risk for developing cutaneous and uterine leiomyomas and aggressive FH-deficient renal cell carcinoma (RCC) with a papillary histology. Due to a disrupted tricarboxylic acid cycle, FH-deficient kidney cancers rely on aerobic glycolysis for energy production, potentially creating compensatory metabolic vulnerabilities. This study conducted a high-throughput drug screen in HLRCC cell lines, which identified a critical dependency on nicotinamide adenine dinucleotide (NAD), a redox cofactor produced by the biosynthetic enzyme nicotinamide phosphoribosyltransferase (NAMPT). Human HLRCC tumors and HLRCC-derived cell lines exhibited elevated NAMPT expression compared with controls. FH-deficient HLRCC cells, but not FH-restored HLRCC or normal kidney cells, were sensitive to NAMPT inhibition. HLRCC cell line viability was significantly decreased in both 2D and 3D in vitro cultures in response to the clinically relevant NAMPT inhibitor OT-82. NAMPT inhibition in vitro significantly decreased the total amount of NAD+, NADH, NADP, NADPH, and poly-ADP-ribose levels, and the effects of NAMPT inhibition could be rescued by the downstream NAD precursor nicotinamide mononucleotide (NMN), confirming the on-target activity of OT-82. Moreover, NAMPT inhibition by OT-82 in two HLRCC xenograft models resulted in severely reduced tumor growth. OT-82 treatment of HLRCC xenograft tumors in vivo inhibited glycolytic flux as demonstrated by reduced lactate/pyruvate ratio in hyperpolarized 13C-pyruvate magnetic resonance spectroscopic imaging experiments. Overall, our data define NAMPT inhibition as a potential therapeutic approach for FH-deficient HLRCC-associated RCC.
©2024 American Association for Cancer Research.
Figures


References
-
- Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 2002;30:406–10. - PubMed
-
- Linehan WM, Schmidt LS, Crooks DR, Wei D, Srinivasan R, Lang M, et al. The metabolic basis of kidney cancer. Cancer Discov 2019;9:1006–21. - PubMed
-
- Yang Y, Valera VA, Padilla-Nash HM, Sourbier C, Vocke CD, Vira MA, et al. UOK 262 cell line, fumarate hydratase deficient (FH−/FH−) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet Cytogenet 2010;196:45–55. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

