Ralstonia solanacearum Infection Drives the Assembly and Functional Adaptation of Potato Rhizosphere Microbial Communities
- PMID: 39397304
- PMCID: PMC11471926
- DOI: 10.5423/PPJ.OA.06.2024.0086
Ralstonia solanacearum Infection Drives the Assembly and Functional Adaptation of Potato Rhizosphere Microbial Communities
Abstract
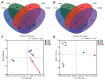
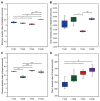
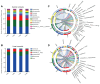
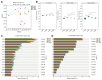
Bacterial wilt caused by Ralstonia solanacearum is a destructive disease that affects potato production, leading to severe yield losses. Currently, little is known about the changes in the assembly and functional adaptation of potato rhizosphere microbial communities during different stages of R. solanacearum infection. In this study, using amplicon and metagenomic sequencing approaches, we analyzed the changes in the composition and functions of bacterial and fungal communities in the potato rhizosphere across four stages of R. solanacearum infection. The results showed that R. solanacearum infection led to significant changes in the composition and functions of bacterial and fungal communities in the potato rhizosphere, with various microbial properties (including α,β-diversity, species composition, and community ecological functions) all being driven by R. solanacearum infection. The relative abundance of some beneficial microorganisms in the potato rhizosphere, including Firmicutes, Bacillus, Pseudomonas, and Mortierella, decreased as the duration of infection increased. Moreover, the related microbial communities played a significant role in basic metabolism and signal transduction; however, the functions involved in soil C, N, and P transformation weakened. This study provides new insights into the dynamic changes in the composition and functions of potato rhizosphere microbial communities at different stages of R. solanacearum infection to adapt to the growth promotion or disease suppression strategies of host plants, which may provide guidance for formulating future strategies to regulate microbial communities for the integrated control of soil-borne plant diseases.
Keywords: functional adaptability; potato; rhizosphere microbial communities.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
Continuous cropping disorders of eggplants (Solanum melongena L.) and tomatoes (Solanum lycopersicum L.) in suburban agriculture: Microbial structure and assembly processes.Sci Total Environ. 2024 Jan 20;909:168558. doi: 10.1016/j.scitotenv.2023.168558. Epub 2023 Nov 17. Sci Total Environ. 2024. PMID: 37979870
-
Effects of the invasion of Ralstonia solanacearum on soil microbial community structure in Wuhan, China.mSphere. 2024 Feb 28;9(2):e0066523. doi: 10.1128/msphere.00665-23. Epub 2024 Jan 17. mSphere. 2024. PMID: 38231250 Free PMC article.
-
Microbial Cross-Talk: Dissecting the Core Microbiota Associated With Flue-Cured Tobacco (Nicotiana tabacum) Plants Under Healthy and Diseased State.Front Microbiol. 2022 Apr 14;13:845310. doi: 10.3389/fmicb.2022.845310. eCollection 2022. Front Microbiol. 2022. PMID: 35495684 Free PMC article.
-
Plant-Microbe Interaction: Mining the Impact of Native Bacillus amyloliquefaciens WS-10 on Tobacco Bacterial Wilt Disease and Rhizosphere Microbial Communities.Microbiol Spectr. 2022 Aug 31;10(4):e0147122. doi: 10.1128/spectrum.01471-22. Epub 2022 Aug 1. Microbiol Spectr. 2022. PMID: 35913211 Free PMC article.
-
Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era.Mol Plant Pathol. 2013 Sep;14(7):651-62. doi: 10.1111/mpp.12038. Epub 2013 May 30. Mol Plant Pathol. 2013. PMID: 23718203 Free PMC article. Review.
Cited by
-
Changes in the community composition and function of the rhizosphere microbiome in tobacco plants with Fusarium root rot.Front Microbiol. 2025 Apr 9;16:1512694. doi: 10.3389/fmicb.2025.1512694. eCollection 2025. Front Microbiol. 2025. PMID: 40291803 Free PMC article.
References
-
- Ahmed W., Dai Z., Zhang J., Li S., Ahmed A., Munir S., Liu Q., Tan Y., Ji G., Zhao Z. Plant-microbe interaction: mining the impact of native Bacillus amyloliquefaciens WS-10 on tobacco bacterial wilt disease and rhizosphere microbial communities. Microbiol. Spectr. 2022b;10:e01471–22. - PMC - PubMed
-
- Ahmed W., Zhou G., Yang J., Munir S., Ahmed A., Liu Q., Zhao Z., Ji G. Bacillus amyloliquefaciens WS-10 as a potential plant growth-promoter and biocontrol agent for bacterial wilt disease of flue-cured tobacco. Egypt. J. Biol. Pest Control. 2022c;32:25.
-
- Anwar M. M., Parveen A., Hossain M. M., Mahamud N. U., Roy R. K. Efficacy of fungicides in controlling late blight of potato. Progr. Agric. 2015;26:103–108.
-
- Arevalo P., VanInsberghe D., Elsherbini J., Gore J., Polz M. F. A reverse ecology approach based on a biological definition of microbial populations. Cell. 2019;178:820–834. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

