Efficacy and safety of immunotherapy combined with chemotherapy in patients with ES-SCLC: A systematic review and network meta-analysis of RCTs and RWSs
- PMID: 39397360
- PMCID: PMC11586137
- DOI: 10.1111/1759-7714.15458
Efficacy and safety of immunotherapy combined with chemotherapy in patients with ES-SCLC: A systematic review and network meta-analysis of RCTs and RWSs
Abstract
Objectives: To evaluate the efficacy and safety of programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors in the treatment of extensive-stage small-cell lung cancer (ES-SCLC), we conducted a systematic review and meta-analysis that included randomized controlled trials (RCTs) and real-world studies (RWS).
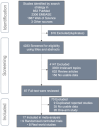
Methods: By scanning PubMed, Web of science, Embase, and other relevant clinical information public databases, nine RCTs and eight RWSs involving 5205 patients were included in the study. We directly compared the differences between chemotherapy and PD-1/PD-L1 inhibitors plus chemotherapy, and determined the optimal treatment strategy through network meta-analysis (NMA).
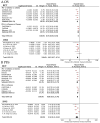
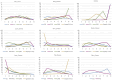
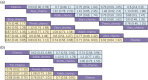
Results: Compared to chemotherapy, the addition of PD-1/PD-L1 inhibitors significantly improves the overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) in SCLC patients. Regarding safety, both RCTs and RWSs indicated no significant difference in grade 3-4 adverse events between chemotherapy and chemoimmunotherapy. NMA showed serplulimab plus chemotherapy (Serp_Chemo) appears to provide the best OS, PFS, and ORR benefit, while nivolumab plus chemotherapy shows higher toxicity than other regimens. In subgroup analysis, for elderly patients (age ≥65) and non-elderly (age <65) patients, the most promising quality regimens for achieving better OS extension are atezolizumab plus chemotherapy (Atez_Chemo) and Serp_Chemo, respectively. For patients with PD-L1 ≥ 1% and lactate dehydrogenase (LDH) > upper limit of normal (ULN), there is no apparent OS benefit from immune therapy.
Conclusions: In ES-SCLC treatment, adding PD-1/PD-L1 inhibitors to standard chemotherapy improves OS, PFS, and ORR, with Serp_Chemo shows the most promise. Atez_Chemo and Serp_Chemo provided better survival for elderly and non-elderly patients, respectively.
Keywords: elderly; extensive stage small cell lung cancer; immune checkpoint inhibitors; meta‐analysis.
© 2024 The Author(s). Thoracic Cancer published by John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Efficacy and safety of first-line immunotherapy plus chemotherapy in treating patients with extensive-stage small cell lung cancer: a Bayesian network meta-analysis.Front Immunol. 2023 Jun 26;14:1197044. doi: 10.3389/fimmu.2023.1197044. eCollection 2023. Front Immunol. 2023. PMID: 37435087 Free PMC article.
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
Efficacy and safety of first-line PD-1/PD-L1 inhibitors combined with or without anti-angiogenesis therapy for extensive-stage small-cell lung cancer: a network meta-analysis.Ther Adv Med Oncol. 2025 Jun 25;17:17588359251348310. doi: 10.1177/17588359251348310. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40574965 Free PMC article.
-
Multidimensional comparative evaluation of first-line therapies for extensive-stage small cell lung cancer: a systematic review and network meta-analysis of clinical efficacy and safety profiles.BMC Cancer. 2025 Aug 9;25(1):1292. doi: 10.1186/s12885-025-14750-4. BMC Cancer. 2025. PMID: 40783512 Free PMC article.
-
Comparison of Efficacy and Safety of Single and Double Immune Checkpoint Inhibitor-Based First-Line Treatments for Advanced Driver-Gene Wild-Type Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis.Front Immunol. 2021 Aug 16;12:731546. doi: 10.3389/fimmu.2021.731546. eCollection 2021. Front Immunol. 2021. PMID: 34484242 Free PMC article.
References
-
- Cheng Y, Han L, Wu L, et al. LBA9 updated results of first‐line serplulimab versus placebo combined with chemotherapy in extensive‐stage small cell lung cancer: an international multicentre phase III study (ASTRUM‐005). Journal Article; Conference Proceeding. Ann Oncol. 2022;33:S1562. 10.1016/j.annonc.2022.10.351 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

