mARC1 Is the Main Contributor to Metabolic Reduction of N-Hydroxyurea
- PMID: 39397364
- PMCID: PMC11513889
- DOI: 10.1021/acs.jmedchem.4c01148
mARC1 Is the Main Contributor to Metabolic Reduction of N-Hydroxyurea
Abstract
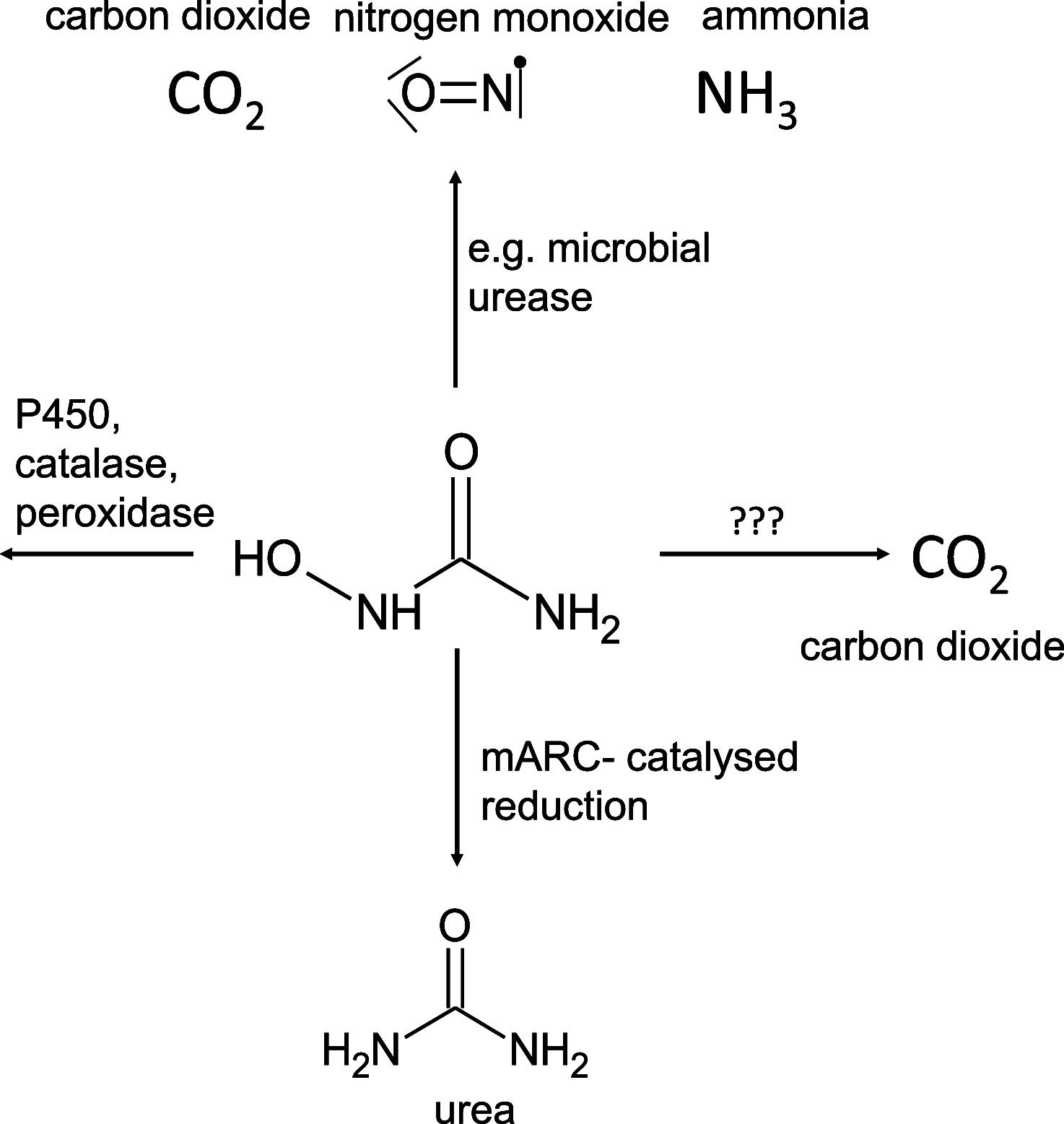
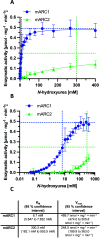
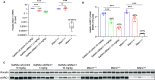
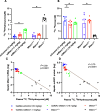
N-Hydroxyurea has been known since the 1960s as an antiproliferative drug and is used both in oncology and for treatment of hematological disorders such as sickle cell anemia where very high daily doses are administered. It is assumed that the cellular effect of N-hydroxyurea is caused by inhibition of ribonucleotide reductase, while alternative mechanisms, e.g., generation of nitric oxide, have also been proposed. Despite its many therapeutic applications, the metabolism of hydroxyurea is largely unexplored. The major elimination pathway of N-hydroxyurea is the reduction to urea. Since the mitochondrial amidoxime reducing component (mARC) is known for its N-reductive activity, we investigated the reduction of NHU by this enzyme system. This study presents in vitro and in vivo evidence that this reductive biotransformation is specifically mediated by the mARC1. Inactivation by mARC1 is a possible explanation for the high doses of NHU required for treatment.
Conflict of interest statement
The authors declare the following competing financial interest(s): All authors are presently employed by or have received research funding from Boehringer Ingelheim.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

