Bioinformatics approaches for studying molecular sex differences in complex diseases
- PMID: 39397573
- PMCID: PMC11471957
- DOI: 10.1093/bib/bbae499
Bioinformatics approaches for studying molecular sex differences in complex diseases
Abstract
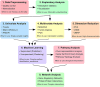
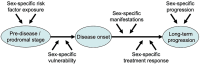
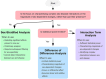
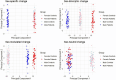
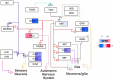
Many complex diseases exhibit pronounced sex differences that can affect both the initial risk of developing the disease, as well as clinical disease symptoms, molecular manifestations, disease progression, and the risk of developing comorbidities. Despite this, computational studies of molecular data for complex diseases often treat sex as a confounding variable, aiming to filter out sex-specific effects rather than attempting to interpret them. A more systematic, in-depth exploration of sex-specific disease mechanisms could significantly improve our understanding of pathological and protective processes with sex-dependent profiles. This survey discusses dedicated bioinformatics approaches for the study of molecular sex differences in complex diseases. It highlights that, beyond classical statistical methods, approaches are needed that integrate prior knowledge of relevant hormone signaling interactions, gene regulatory networks, and sex linkage of genes to provide a mechanistic interpretation of sex-dependent alterations in disease. The review examines and compares the advantages, pitfalls and limitations of various conventional statistical and systems-level mechanistic analyses for this purpose, including tailored pathway and network analysis techniques. Overall, this survey highlights the potential of specialized bioinformatics techniques to systematically investigate molecular sex differences in complex diseases, to inform biomarker signature modeling, and to guide more personalized treatment approaches.
Keywords: bioinformatics; biomarker signature modeling; complex diseases; molecular sex differences; pathway and network analysis; personalized medicine.
© The Author(s) 2024. Published by Oxford University Press.
Figures


References
-
- Groban L, Lindsey SH, Wang H. et al. Chapter 5 - sex and gender differences in cardiovascular disease. Sex Differences in Physiology 2016;1:61–87. 10.1016/B978-0-12-802388-4.00005-7. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

