Physiological liver microtissue 384-well microplate system for preclinical hepatotoxicity assessment of therapeutic small molecule drugs
- PMID: 39397666
- PMCID: PMC11664105
- DOI: 10.1093/toxsci/kfae123
Physiological liver microtissue 384-well microplate system for preclinical hepatotoxicity assessment of therapeutic small molecule drugs
Abstract
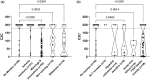
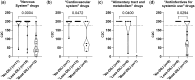
Hepatotoxicity can lead to the discontinuation of approved or investigational drugs. The evaluation of the potential hepatoxicity of drugs in development is challenging because current models assessing this adverse effect are not always predictive of the outcome in human beings. Cell lines are routinely used for early hepatotoxicity screening, but to improve the detection of potential hepatotoxicity, in vitro models that better reflect liver morphology and function are needed. One such promising model is human liver microtissues. These are spheroids made of primary human parenchymal and nonparenchymal liver cells, which are amenable to high throughput screening. To test the predictivity of this model, the cytotoxicity of 152 FDA (US Food & Drug Administration)-approved small molecule drugs was measured as per changes in ATP content in human liver microtissues incubated in 384-well microplates. The results were analyzed with respect to drug label information, drug-induced liver injury (DILI) concern class, and drug class. The threshold IC50ATP-to-Cmax ratio of 176 was used to discriminate between safe and hepatotoxic drugs. "vMost-DILI-concern" drugs were detected with a sensitivity of 72% and a specificity of 89%, and "vMost-DILI-concern" drugs affecting the nervous system were detected with a sensitivity of 92% and a specificity of 91%. The robustness and relevance of this evaluation were assessed using a 5-fold cross-validation. The good predictivity, together with the in vivo-like morphology of the liver microtissues and scalability to a 384-well microplate, makes this method a promising and practical in vitro alternative to 2D cell line cultures for the early hepatotoxicity screening of drug candidates.
Keywords: DILI; drug safety; drug-induced liver injury; microphysiological system; spheroid cultures.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology.
Conflict of interest statement
LF, MT, KS, HV, KK, MVC, and BGHF are currently employed by InSphero AG which is active in the business of in vitro hepatotoxicity assessment using human liver microtissues. NJH, FW, NZ-B, WT, and MC have no conflicts of interest.
Figures



References
-
- Antineoplastic Agents. 2012. LiverTox. [accessed 2024 Apr 22]. https://www.ncbi.nlm.nih.gov/books/NBK548022/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

