A seamless Phase I/II platform design with a time-to-event efficacy endpoint for potential COVID-19 therapies
- PMID: 39397762
- PMCID: PMC11577684
- DOI: 10.1177/09622802241288348
A seamless Phase I/II platform design with a time-to-event efficacy endpoint for potential COVID-19 therapies
Abstract
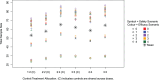
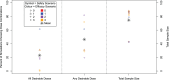
In the search for effective treatments for COVID-19, the initial emphasis has been on re-purposed treatments. To maximize the chances of finding successful treatments, novel treatments that have been developed for this disease in particular, are needed. In this article, we describe and evaluate the statistical design of the AGILE platform, an adaptive randomized seamless Phase I/II trial platform that seeks to quickly establish a safe range of doses and investigates treatments for potential efficacy. The bespoke Bayesian design (i) utilizes randomization during dose-finding, (ii) shares control arm information across the platform, and (iii) uses a time-to-event endpoint with a formal testing structure and error control for evaluation of potential efficacy. Both single-agent and combination treatments are considered. We find that the design can identify potential treatments that are safe and efficacious reliably with small to moderate sample sizes.
Keywords: Adaptive platform trial; COVID-19; dose-escalation; randomized; seamless; time-to-improvement.
Conflict of interest statement
Declaration of conflicting interestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Beigel JH, Tomashek KM, Dodd LE. et al.. Remdesivir for the treatment of COVID-19. N Engl J Med 2020; 383: 1813–1836. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

