USP5 promotes tumor progression by stabilizing SLUG in bladder cancer
- PMID: 39397799
- PMCID: PMC11467842
- DOI: 10.3892/ol.2024.14705
USP5 promotes tumor progression by stabilizing SLUG in bladder cancer
Abstract
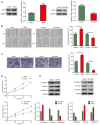
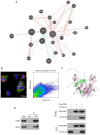
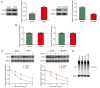
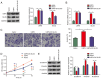
Bladder cancer ranks as the second most prevalent urology malignancy globally. Invasive metastasis is a significant contributor to mortality among patients with bladder cancer, yet the underlying mechanisms remain elusive. Deubiquitinases are pivotal in carcinogenesis, with USP5 implicated in the malignant progression of hepatocellular carcinoma, colorectal cancer and non-small cell lung cancer. The present study assessed the role and mechanism of ubiquitin-specific proteinase 5 (USP5) in the malignant progression of bladder cancer. The association between USP5 expression and bladder cancer prognosis and stage was analyzed using The Cancer Genome Atlas database. Moreover, to elucidate the role of USP5 in bladder cancer, USP5 overexpression and knockdown cell lines were established using T24 cells. Cell viability, proliferation and migration were assessed using Cell Counting Kit-8, Transwell and scratch assays, respectively. Cyclohexanamide was used to evaluate the effect of USP5 expression on Snail family zinc finger 2 (SLUG) stability. Immunoprecipitation and immunofluorescence co-localization were utilized to probe the interaction between USP5 and SLUG. Changes in mRNA and protein levels were assessed using reverse transcription-quantitative PCR and western blotting, respectively. The results revealed that patients with bladder cancer with high USP5 expression had significantly shorter survival (P<0.05) and a higher clinicopathologic stage (P<0.05) than those with low USP5 expression. T24 cells overexpressing USP5 demonstrated significantly increased proliferation (P<0.05), invasion (P<0.05) and expression of epithelial-mesenchymal transition markers (P<0.05); whereas T24 cells with knocked-down USP5 expression revealed significantly reduced proliferation (P<0.05), invasion (P<0.05) and epithelial-mesenchymal transition markers (P<0.05). Immunoprecipitation experiments demonstrated the binding of USP5 to SLUG in bladder cancer cells, with further analysis revealing that USP5 upregulated protein levels of SLUG by inhibiting its ubiquitination. Furthermore, the treatment of bladder cancer cells with Degrasyn, a USP5 inhibitor, was associated with a significant inhibition of the proliferation (P<0.05) and invasion (P<0.05) of T24 cells. In conclusion, the findings of the present study underscore the role of USP5 in promoting the malignant progression of bladder cancer through the stabilization of SLUG. Targeting USP5 holds promise as a strategy for inhibiting bladder cancer progression.
Keywords: Snail family zinc finger 2; bladder cancer; epithelial-mesenchymal transition; ubiquitin-specific proteinase 5.
Copyright: © 2024 Wan et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
