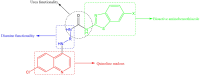
In vitro antiplasmodium and antitrypanosomal activities, β-haematin formation inhibition, molecular docking and DFT computational studies of quinoline-urea-benzothiazole hybrids
- PMID: 39397937
- PMCID: PMC11471183
- DOI: 10.1016/j.heliyon.2024.e38434
In vitro antiplasmodium and antitrypanosomal activities, β-haematin formation inhibition, molecular docking and DFT computational studies of quinoline-urea-benzothiazole hybrids
Erratum in
-
Corrigendum to "In vitro antiplasmodium and antitrypanosomal activities, β-haematin formation inhibition, molecular docking and DFT computational studies of quinoline-urea-benzothiazole hybrids" [Heliyon Volume 10, Issue 19, October 2024, Article e38434].Heliyon. 2024 Nov 15;10(23):e40195. doi: 10.1016/j.heliyon.2024.e40195. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39759860 Free PMC article.
Abstract
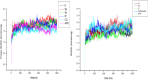
Quinoline-urea-benzothiazole hybrids exhibited low to sub-micromolar in vitro activities against the Plasmodium falciparum (P. falciparum) 3D7 chloroquine (CQ)-sensitive strain, with compounds 5a, 5b and 5f showing activities ranging from 0.33 to 0.97 μM. Against the formation of β-haematin, the majority of the tested compounds were comparable to the reference drug, chloroquine (CQ), with compounds 5c (IC50 = 9.55 ± 0.62 μM) and 5h (IC50 = 9.73 ± 1.38 μM), exhibiting slightly better in vitro efficacy than CQ. The hybrids also exhibited low micromolar to submicromolar activities against Trypanosoma brucei brucei, with 5j-5k being comparable to the reference drug, pentamidine. Compound 5b displayed higher in silico binding energy than CQ when docked against P. falciparum dihydroorotate dehydrogenase enzyme. Compounds 5j and 5k showed higher binding energies than pentamidine within the trypanothione reductase enzyme binding pocket. The root means square deviations of the hit compounds 5b, 5j and 5k were stable throughout the 100 ns simulation period. Post-molecular dynamics MMGBSA binding free energies showed that the selected hybrids bind spontaneously to the respective enzymes. The DFT investigation revealed that the compounds have regions that can bind to the electropositive and electronegative sites of the proteins.
Keywords: Ligand-receptor complex and density functional theory (DFT) studies; Molecular docking; Quinoline-urea-benzothizole hybrids; β-haematin formation inhibition.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- World Health Organization . WHO; Geneva: 2023. Global Report on Neglected Tropical Diseases 2023.https://www.who.int/publications/i/item/9789240067295
-
- Papagni R., Novara R., Minardi M.L., Frallonardo L., Panico G.G., Pallara E., Cotugno S., Bartoli T.A., Guido G., De Vita E., Ricciardi A., Totaro V., Camporeale M., Segala F.S., Bavaro D.F., Patti G., Brindicci G., Pellegrino C., Mariani M.F., Putoto G., Sarmati L., Castellani C., Saracino A., Di Gennaro F., Nicastri E. Human African Trypanosomiasis (sleeping sickness): current knowledge and future challenges. Front. in Trop. Dis. 2023;4 doi: 10.1186/s43088-022-00313-03389/fitd.2023.1087003. - DOI
-
- Monti L., Wang S.C., Oukoloff K., Smith A.B., 3rd, Brunden K.R., Caffrey C.R., Ballatore C. Brain-penetrant triazolopyrimidine and phenylpyrimidine microtubule stabilizers as potential leads to treat human african trypanosomiasis. ChemMedChem. 2018;13:1751. doi: 10.1002/cmdc.201800404. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

