Enhanced phosphorus adsorption using modified drinking water treatment residues: A comparative analysis of powder and alginate bead forms
- PMID: 39397955
- PMCID: PMC11470402
- DOI: 10.1016/j.heliyon.2024.e38144
Enhanced phosphorus adsorption using modified drinking water treatment residues: A comparative analysis of powder and alginate bead forms
Abstract
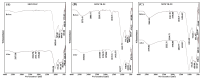
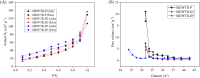
This study provides an analysis of the phosphorus adsorption efficacy of three modified drinking water treatment residues (MDWTRs): MDWTR-P (powdered form), MDWTR-D2, and MDWTR-D5 (alginate bead-entrapped forms with bead diameters of 2 mm and 5 mm, respectively). The preparation process involved washing and drying the drinking water treatment residue, followed by grinding and sieving to achieve particle sizes below 90 μm. The residue was then incinerated at 600 °C in oxygen-limited conditions. Subsequently, the MDWTR was formulated into alginate beads by mixing with sodium alginate and FeCl3 solutions, resulting in spherical particles of specified diameters. The evaluation of surface area, pore volume, pore size, and CHN concentration revealed that MDWTR-D5 possesses the largest surface area (284.7 m2 g-1) and highest micropore volume (0.04 cm3 g-1), indicating a greater capacity for adsorption. SEM-EDS analysis demonstrated significant compositional changes post-treatment, particularly elevated phosphorus levels, confirming effective adsorption. Metal content analysis indicated high aluminum levels in MDWTR-P and increased iron content in MDWTR-D5. Toxicity Characteristic Leaching Procedure (TCLP) and in vitro bioaccessibility (IVBA) tests confirmed the non-hazardous nature of all MDWTRs, ensuring their safety for environmental applications. Kinetic analyses using pseudo-first-order, pseudo-second-order, and intraparticle diffusion models highlighted the superior performance of MDWTR-D5, with the highest equilibrium adsorption capacity and initial adsorption rate across all tested concentrations, suggesting both high efficiency and rapid adsorption potential. Further validation using Langmuir and Freundlich isotherms revealed MDWTR-D5's highest monolayer adsorption capacity (22.88 mg g-1) and Freundlich adsorption capacity parameter (6.97 mg g-1). Statistical analysis via one-way ANOVA confirmed significant differences in phosphorus concentrations among the MDWTRs samples (p-value <0.001), consistently underscoring MDWTR-D5's superior adsorption performance. These findings highlight MDWTR-D5's potential as an effective adsorbent for phosphorus removal in wastewater treatment, emphasizing its applicability in environmental remediation strategies.
Keywords: Adsorption isotherms; Adsorption kinetics; In vitro bioaccessibility (IVBA); Modified drinking water treatment residues (MDWTR); Phosphorus adsorption; Toxicity characteristic leaching procedure (TCLP); Wastewater treatment.
© 2024 The Authors.
Conflict of interest statement
The authors declare that none of the work reported in this study could have been influenced by any known competing financial interests or personal relationships.
Figures


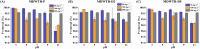


References
-
- Jung K.W., Hwang M.J., Kim K., Ahn K.H. Adsorption of phosphate from aqueous solution on to pyrolyzed drinking water treatment residuals: statistical process optimization, equilibrium, and kinetic analysis. Environ. Prog. Sustain. Energy. 2014;35(4):1035–1046. doi: 10.1002/ep.12319. - DOI
-
- Wildemeersch M., Tang S., Ermolieva T., Ermoliev Y., Rovenskaya E., Obersteiner M. Containing the risk of phosphorus pollution in agricultural watersheds. Sustainability. 2020;14(3):1717. doi: 10.3390/su14031717. - DOI
LinkOut - more resources
Full Text Sources

