Cabozantinib prevents the progression of metabolic dysfunction-associated steatohepatitis by inhibiting the activation of hepatic stellate cell and macrophage and attenuating angiogenic activity
- PMID: 39398008
- PMCID: PMC11470516
- DOI: 10.1016/j.heliyon.2024.e38647
Cabozantinib prevents the progression of metabolic dysfunction-associated steatohepatitis by inhibiting the activation of hepatic stellate cell and macrophage and attenuating angiogenic activity
Abstract
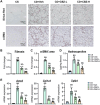
Cabozantinib, a multiple tyrosine kinase inhibitor targeting AXL, vascular endothelial growth factor receptor (VEGFR), and MET, is used clinically to treat certain cancers, including hepatocellular carcinoma. This study aimed to assess the impact of cabozantinib on liver fibrosis and hepatocarcinogenesis in a rat model of metabolic dysfunction-associated steatohepatitis (MASH). MASH-based liver fibrosis and hepatocarcinogenesis were induced in rats by feeding them a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) for eight and 16 weeks, respectively. Cabozantinib (1 or 2 mg/kg, daily) was administered concurrently with the diet in the fibrosis model and after eight weeks in the carcinogenesis model. Treatment with cabozantinib significantly attenuated hepatic inflammation and fibrosis without affecting hepatocyte steatosis and ballooning in CDAHFD-fed rats. Cabozantinib-treated rats exhibited a marked reduction in α-smooth muscle actin+ activated hepatic stellate cell (HSC) expansion, CD68+ macrophage infiltration, and CD34+ pathological angiogenesis, along with reduced hepatic AXL, VEGF, and VEGFR2 expression. Consistently, cabozantinib downregulated the hepatic expression of profibrogenic markers (Acta2, Col1a1, Tgfb1), inflammatory cytokines (Tnfa, Il1b, Il6), and proangiogenic markers (Vegfa, Vwf, Ang2). In a cell-based assay of human activated HSCs, cabozantinib inhibited Akt activation induced by GAS6, a ligand of AXL, leading to reduced cell proliferation and profibrogenic activity. Cabozantinib also suppressed lipopolysaccharide-induced proinflammatory responses in human macrophages, VEGFA-induced collagen expression and proliferation in activated HSCs, and VEGFA-stimulated proliferation in vascular endothelial cells. Meanwhile, administration of cabozantinib did not affect Ki67+ hepatocyte proliferation or serum albumin levels, indicating no negative impact on regenerative capacity. Treatment with cabozantinib also reduced the placental glutathione transferase+ preneoplastic lesions in CDAHFD-fed rats. In conclusion, cabozantinib shows promise as a novel option for preventing MASH progression.
Keywords: Angiogenesis; Hepatocarcinogenesis; Inflammation; Liver fibrosis; MASH.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

