DNA-PKcs inhibition improves sequential gene insertion of the full-length CFTR cDNA in airway stem cells
- PMID: 39398224
- PMCID: PMC11470261
- DOI: 10.1016/j.omtn.2024.102339
DNA-PKcs inhibition improves sequential gene insertion of the full-length CFTR cDNA in airway stem cells
Abstract
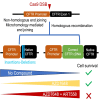
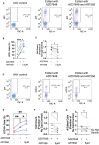
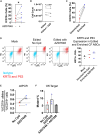
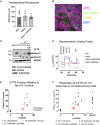
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Although many people with CF (pwCF) are treated using CFTR modulators, some are non-responsive due to their genotype or other uncharacterized reasons. Autologous airway stem cell therapies, in which the CFTR cDNA has been replaced, may enable a durable therapy for all pwCF. Previously, CRISPR-Cas9 with two AAVs was used to sequentially insert two-halves of the CFTR cDNA and an enrichment cassette into the CFTR locus. However, the editing efficiency was <10% and required enrichment to restore CFTR function. Further improvement in gene insertion may enhance cell therapy production. To improve CFTR cDNA insertion in human airway basal stem cells (ABCs), we evaluated the use of the small molecules AZD7648 and ART558, which inhibit non-homologous end-joining (NHEJ) and micro-homology mediated end-joining (MMEJ). Adding AZD7648 alone improved gene insertion by 2- to 3-fold. Adding both ART558 and AZD7648 improved gene insertion but induced toxicity. ABCs edited in the presence of AZD7648 produced differentiated airway epithelial sheets with restored CFTR function after enrichment. Adding AZD7648 did not increase off-target editing. Further studies are necessary to validate if AZD7648 treatment enriches cells with oncogenic mutations.
Keywords: CFTR genome editing; CFTR super-exon; DNA-PKcs inhibition; MT: RNA/DNA Editing; airway stem cell therapy; cystic fibrosis; sequential gene insertion; universal CFTR correction.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Update of
-
DNA-PKcs Inhibition Improves Sequential Gene Insertion of the Full-Length CFTR cDNA in Airway Stem Cells.bioRxiv [Preprint]. 2024 Aug 12:2024.08.12.607571. doi: 10.1101/2024.08.12.607571. bioRxiv. 2024. Update in: Mol Ther Nucleic Acids. 2024 Sep 16;35(4):102339. doi: 10.1016/j.omtn.2024.102339. PMID: 39185207 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources

