Precision and efficacy of RNA-guided DNA integration in high-expressing muscle loci
- PMID: 39398225
- PMCID: PMC11466678
- DOI: 10.1016/j.omtn.2024.102320
Precision and efficacy of RNA-guided DNA integration in high-expressing muscle loci
Abstract
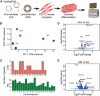
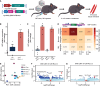
Gene replacement therapies primarily rely on adeno-associated virus (AAV) vectors for transgene expression. However, episomal expression can decline over time due to vector loss or epigenetic silencing. CRISPR-based integration methods offer promise for long-term transgene insertion. While the development of transgene integration methods has made substantial progress, identifying optimal insertion loci remains challenging. Skeletal muscle is a promising tissue for gene replacement owing to low invasiveness of intramuscular injections, relative proportion of body mass, the multinucleated nature of muscle, and the potential for reduced adverse effects. Leveraging endogenous promoters in skeletal muscle, we evaluated two highly expressing loci using homology-independent targeted integration (HITI) to integrate reporter or therapeutic genes in mouse myoblasts and skeletal muscle tissue. We hijacked the muscle creatine kinase (Ckm) and myoglobin (Mb) promoters by co-delivering CRISPR-Cas9 and a donor plasmid with promoterless constructs encoding green fluorescent protein (GFP) or human Factor IX (hFIX). Additionally, we deeply profiled our genome and transcriptome outcomes from targeted integration and evaluated the safety of the proposed sites. This study introduces a proof-of-concept technology for achieving high-level therapeutic gene expression in skeletal muscle, with potential applications in targeted integration-based medicine and synthetic biology.
Keywords: CRISPR; MT: RNA/DNA Editing; endogenous-promoter; gene editing; homology-independent targeted integration (HITI); integration; muscle; muscle-specific promoters; overexpression; sequencing.
© 2024 The Author(s).
Conflict of interest statement
M.H.P. and C.E.N. are named inventors on patents and patent applications related to genome editing.
Figures





Update of
-
Precision and efficacy of RNA-guided DNA integration in high-expressing muscle loci.bioRxiv [Preprint]. 2024 Mar 19:2024.03.18.582796. doi: 10.1101/2024.03.18.582796. bioRxiv. 2024. Update in: Mol Ther Nucleic Acids. 2024 Sep 02;35(4):102320. doi: 10.1016/j.omtn.2024.102320. PMID: 38562818 Free PMC article. Updated. Preprint.
References
-
- Niemeyer G.P., Herzog R.W., Mount J., Arruda V.R., Tillson D.M., Hathcock J., van Ginkel F.W., High K.A., Lothrop C.D., Jr. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. - DOI - PMC - PubMed
-
- Nathwani A.C., Reiss U., Tuddenham E., Chowdary P., McIntosh J., Riddell A., Pie J., Mahlangu J.N., Recht M., Shen Y.-M., et al. Adeno-Associated Mediated Gene Transfer for Hemophilia B:8 Year Follow up and Impact of Removing “Empty Viral Particles” on Safety and Efficacy of Gene Transfer. Blood. 2018;132:491. doi: 10.1182/blood-2018-99-118334. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

