In situ evolution of electrocatalysts for enhanced electrochemical nitrate reduction under realistic conditions
- PMID: 39398413
- PMCID: PMC11470436
- DOI: 10.1016/j.ese.2024.100492
In situ evolution of electrocatalysts for enhanced electrochemical nitrate reduction under realistic conditions
Abstract
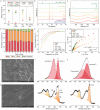
Electrochemical nitrate reduction to ammonia (ENRA) is gaining attention for its potential in water remediation and sustainable ammonia production, offering a greener alternative to the energy-intensive Haber-Bosch process. Current research on ENRA is dedicated to enhancing ammonia selectively and productivity with sophisticated catalysts. However, the performance of ENRA and the change of catalytic activity in more complicated solutions (i.e., nitrate-polluted groundwater) are poorly understood. Here we first explored the influence of Ca2+ and bicarbonate on ENRA using commercial cathodes. We found that the catalytic activity of used Ni or Cu foam cathodes significantly outperforms their pristine ones due to the in situ evolution of new catalytic species on used cathodes during ENRA. In contrast, the nitrate conversion performance with nonactive Ti or Sn cathode is less affected by Ca2+ or bicarbonate because of their original poor activity. In addition, the coexistence of Ca2+ and bicarbonate inhibits nitrate conversion by forming scales (CaCO3) on the in situ-formed active sites. Likewise, ENRA is prone to fast performance deterioration in treating actual groundwater over continuous flow operation due to the presence of hardness ions and possible organic substances that quickly block the active sites toward nitrate reduction. Our work suggests that more work is required to ensure the long-term stability of ENRA in treating natural nitrate-polluted water bodies and to leverage the environmental relevance of ENRA in more realistic conditions.
Keywords: Ammonium; Cathodic corrosion; Groundwater; Hardness ions; In situ activation.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
The Duality of Ion Effects: How Cation Precipitation Sites and Anion Interactions Shape Electrochemical Nitrate Reduction.ACS Appl Mater Interfaces. 2025 Jul 9;17(27):39032-39040. doi: 10.1021/acsami.5c05397. Epub 2025 Jun 27. ACS Appl Mater Interfaces. 2025. PMID: 40576377
-
Graphene Oxide-Anchored Cu-Co Catalysts for Efficient Electrochemical Nitrate Reduction.Materials (Basel). 2025 May 26;18(11):2495. doi: 10.3390/ma18112495. Materials (Basel). 2025. PMID: 40508493 Free PMC article.
-
Innovative Strategies for Designing Metallic Compounds in Electrocatalytic Nitrate-to-Ammonia Reduction.ChemSusChem. 2025 Jun 2;18(11):e202500229. doi: 10.1002/cssc.202500229. Epub 2025 Apr 10. ChemSusChem. 2025. PMID: 40105903 Review.
-
Recent progress on Ti-based catalysts in the electrochemical synthesis of ammonia.Nanoscale. 2024 Sep 26;16(37):17300-17323. doi: 10.1039/d4nr02852j. Nanoscale. 2024. PMID: 39240163 Review.
-
A GO regulated bimetallic CoFe catalyst for efficient electrochemical nitrate reduction to ammonia under acidic conditions.Chem Commun (Camb). 2024 Nov 7;60(90):13231-13234. doi: 10.1039/d4cc04897k. Chem Commun (Camb). 2024. PMID: 39445415
References
-
- Smith C., Hill A.K., Torrente-Murciano L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020;13:331–344.
-
- Chen G.-F., Yuan Y., Jiang H., Ren S.-Y., Ding L.-X., Ma L., Wu T., Lu J., Wang H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy. 2020;5:605–613.
-
- Lim J., Fernández C.A., Lee S.W., Hatzell M.C. Ammonia and nitric acid demands for fertilizer use in 2050. ACS Energy Lett. 2021;6:3676–3685.
LinkOut - more resources
Full Text Sources
Miscellaneous

