Improving the endoscopic recognition of early colorectal carcinoma using artificial intelligence: current evidence and future directions
- PMID: 39398448
- PMCID: PMC11466514
- DOI: 10.1055/a-2403-3103
Improving the endoscopic recognition of early colorectal carcinoma using artificial intelligence: current evidence and future directions
Abstract
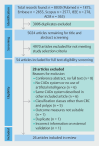
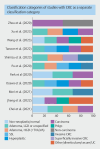
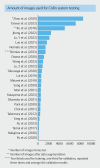
Background and study aims Artificial intelligence (AI) has great potential to improve endoscopic recognition of early stage colorectal carcinoma (CRC). This scoping review aimed to summarize current evidence on this topic, provide an overview of the methodologies currently used, and guide future research. Methods A systematic search was performed following the PRISMA-Scr guideline. PubMed (including Medline), Scopus, Embase, IEEE Xplore, and ACM Digital Library were searched up to January 2024. Studies were eligible for inclusion when using AI for distinguishing CRC from colorectal polyps on endoscopic imaging, using histopathology as gold standard, reporting sensitivity, specificity, or accuracy as outcomes. Results Of 5024 screened articles, 26 were included. Computer-aided diagnosis (CADx) system classification categories ranged from two categories, such as lesions suitable or unsuitable for endoscopic resection, to five categories, such as hyperplastic polyp, sessile serrated lesion, adenoma, cancer, and other. The number of images used in testing databases varied from 69 to 84,585. Diagnostic performances were divergent, with sensitivities varying from 55.0% to 99.2%, specificities from 67.5% to 100% and accuracies from 74.4% to 94.4%. Conclusions This review highlights that using AI to improve endoscopic recognition of early stage CRC is an upcoming research field. We introduced a suggestions list of essential subjects to report in research regarding the development of endoscopy CADx systems, aiming to facilitate more complete reporting and better comparability between studies. There is a knowledge gap regarding real-time CADx system performance during multicenter external validation. Future research should focus on development of CADx systems that can differentiate CRC from premalignant lesions, while providing an indication of invasion depth.
Keywords: Colorectal cancer; Diagnosis and imaging (inc chromoendoscopy, NBI, iSCAN, FICE, CLE...); Endoscopy Lower GI Tract; Image and data processing, documentatiton; Polyps / adenomas / ...; Quality and logistical aspects.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
Conflict of Interest Author FvdS received research support from Olympus, outside the submitted work. Author JB is a consultant for Boston Scientific. Author LM is a consultant for Boston Scientific. Author ES received research support and speakers’ fees from Fujifilm, outside the submitted work. Authors AT, RMS, ND, MS, and PdW declare no conflict of interests for this article.
Figures
References
-
- Breekveldt ECH, Lansdorp-Vogelaar I, Toes-Zoutendijk E et al. Colorectal cancer incidence, mortality, tumour characteristics, and treatment before and after introduction of the faecal immunochemical testing-based screening programme in the Netherlands: a population-based study. Lancet Gastroenterol Hepatol. 2022;7:60–68. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

