Evaluating Faricimab in Treatment-Naive Neovascular Age Related Macular Degeneration: A Retrospective Analysis of Real-World Data
- PMID: 39398467
- PMCID: PMC11470206
- DOI: 10.2147/OPTH.S468458
Evaluating Faricimab in Treatment-Naive Neovascular Age Related Macular Degeneration: A Retrospective Analysis of Real-World Data
Abstract
Purpose: To evaluate the efficiency and safety of Faricimab on treatment-naive neovascular age related macular degeneration (nAMD) in a real world UK clinic.
Patients and methods: This single centre, retrospective note review was conducted on treatment-naive patients with nAMD. The data collected included demographics, best corrected visual acuity (BCVA), central macular thickness (CMT), total retinal fluid (TRF), the presence of intraretinal fluid (IRF) and subretinal fluid (SRF).
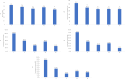
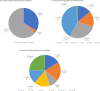
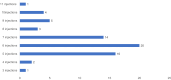
Results: A total of 66 eyes from 62 patients were analysed. The average age was 77 years (range 36-91) and 54% of patients were female. After the first dose of faricimab, the average BCVA improved by 0.05 LogMAR (+2.5 letters), the average CMT decreased by 65.9μm and 41% of patients were found to be inactive. The follow-up intervals after the third loading dose were divided into 2 subsets of 4 and 8 week extensions. The 4 week extension subset saw a smaller improvement in BCVA (+3 letters) than the 8 week extension (+6 letters) while both had an average decrease in CMT by 86.6 μm. The total retinal fluid decreased by 45% and 70.7%, leaving only 30% and 12.2% residual intraretinal fluid (IRF) and 30% and 24.4% residual subretinal fluid (SRF), respectively. Over a ten-month period, the average number of injections received was 6.6, including 3 initial loading doses. There was only one reported case of an adverse event out of 66 eyes (1/66, 1.5%).
Conclusion: Three loading doses of Faricimab appear efficacious and safe for the treatment of nAMD.
Keywords: Faricimab; dosing interval; nAMD; treatment-naïve.
Plain language summary
What is already known on this topic: Faricimab is recombinant humanised bispecific IgG monoclonal antibody which binds and neutralises both VEGF-A and angiopoietin-2 (VEGFA and ANG2).Clinical trials have reported faricimab dosing intervals of up to Q16W. What this study adds: To offer a glimpse into real-world effectiveness and safety of faricimab beyond the constraints of a randomised controlled study in treatment naïve nAMD patients.Presents results derived from implementing a “Treat and Extend” algorithm with faricimab in nAMD management, showcasing its beneficial impact on the strategic planning and execution of patient care. How this research may affect research, practice or policy: Faricimab should be seen as a viable therapy in treatment-naive patients with nAMD.Employing a shortened loading regimen of three doses presents no discernible detriment to treatment efficacy when compared to the established four-dose schedule.
© 2024 Modeste et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Royal College of Ophthalmologists neovascular age related macular degeneration guidelines; 2021.
-
- Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol. 2012;96(5):752–756. Epub 2012 Feb 13. PMID: 22329913; PMCID: PMC3329633. doi: 10.1136/bjophthalmol-2011-301109 - DOI - PMC - PubMed
-
- Kataoka K, Itagaki K, Hashiya N, et al.; for Japan AMD Research Consortium (JARC). Six-month outcomes of switching from aflibercept to faricimab in refractory cases of neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2024;262(1):43–51. doi: 10.1007/s00417-023-06222-x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

