Regulation of p53 by the mitotic surveillance/stopwatch pathway: implications in neurodevelopment and cancer
- PMID: 39398482
- PMCID: PMC11466822
- DOI: 10.3389/fcell.2024.1451274
Regulation of p53 by the mitotic surveillance/stopwatch pathway: implications in neurodevelopment and cancer
Abstract
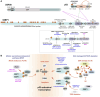
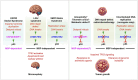
The transcription factor p53 (encoded by TP53) plays diverse roles in human development and disease. While best known for its role in tumor suppression, p53 signaling also influences mammalian development by triggering cell fate decisions in response to a wide variety of stresses. After over 4 decades of study, a new pathway that triggers p53 activation in response to mitotic delays was recently identified. Termed the mitotic surveillance or mitotic stopwatch pathway, the USP28 and 53BP1 proteins activate p53 in response to delayed mitotic progression to control cell fate and promote genomic stability. In this Minireview, I discuss its identification, potential roles in neurodevelopmental disorders and cancer, as well as explore outstanding questions about its function, regulation and potential use as a biomarker for anti-mitotic therapies.
Keywords: 53BP1; USP28; apoptosis; c-MYC; cancer; microcephaly; neurodevelopment; p53.
Copyright © 2024 Stracker.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

