Facilitating microplastic ingestion in aquatic models: A verified protocol for daphnia magna as a trojan horse vector
- PMID: 39398536
- PMCID: PMC11470188
- DOI: 10.1016/j.mex.2024.102973
Facilitating microplastic ingestion in aquatic models: A verified protocol for daphnia magna as a trojan horse vector
Abstract
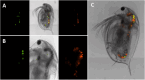
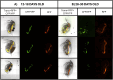
Microplastic pollution poses a significant environmental threat due to its persistence, widespread distribution, and inherent toxic potential. Despite the increasing number of publications in this field, a standardized protocol for the laboratory intake of microplastics by Daphnia magna has yet to be established. In this study, we introduce a verified protocol designed to facilitate the ingestion of microplastic particles (MPs) by D. magna, ranging in size from 5-55 µm. This protocol can be further applied to evaluate the toxicity of MPs on D. magna, a crucial organism model in ecotoxicology. Furthermore, this protocol can be used to assess toxicity of MPs in other aquatic species, such as fish, by using daphnids as a vehicle for ensuring the ingestion of these particles. Consequently, this protocol can be applied to study also one of the most pressing concerns regarding exposure to MPs, the transfer of MPs through different trophic levels, which has a great potential for ecotoxicological studies.•The influence of MPs concentration, duration and exposure dynamics and D. magna age/size in MPs intake were tested.•We have determined the optimal conditions for promoting microplastic ingestion by D. magna.
Keywords: Aquatic Microplastic Ingestion and Transfer Protocol (AMITP); Daphnia magna; Food consumption; Microplastics; Pollution assessment; “Trojan Horse” vector.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- GESAMP . In: IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environment Protection, Reports and Studies GESAMP, 90. Kershaw P.J., editor. 2015. Sources, fate and effects of microplastics in the marine environment: a global assessment; pp. 1–96. - DOI
-
- Da Costa J.P., Nunes A.R., Santos P.S.M., Girão A.V., Duarte A.C., Rocha-Santos T. Degradation of polyethylene microplastics in seawater: insights into the environmental degradation of polymers. J. Environ. Sci. Health - Part A Toxic/Hazardous Substances Environ. Eng. 2018;53(9):866–875. doi: 10.1080/10934529.2018.1455381. - DOI - PubMed
-
- Horton A.A., Walton A., Spurgeon D.J., Lahive E., Svendsen C. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017;586:127–141. doi: 10.1016/J.SCITOTENV.2017.01.190. - DOI - PubMed
LinkOut - more resources
Full Text Sources

