Combination of melatonin-delivered endothelial progenitor cells with S-nitroso-N-acetyl-DL-penicillamine for improving critical limb ischemia in the rat
- PMID: 39398551
- PMCID: PMC11470315
- DOI: 10.62347/OCFT1003
Combination of melatonin-delivered endothelial progenitor cells with S-nitroso-N-acetyl-DL-penicillamine for improving critical limb ischemia in the rat
Abstract
Background: This study tested whether combined shock wave (SW)-facilitated melatonin (Mel) delivered into endothelial progenitor cells (EPCs) (EPCSW-Mel) plus S-nitroso-N-acetyl-DL-penicillamine (SNAP) was superior to merely one modality alone for improving critical limb ischemia (CLI) in rats.
Methods: SD rats (n = 50) were equally categorized into group 1 (sham-control), group 2 (CLI), group 3 (CLI + SNAP), group 4 (CLI + EPCSW-Mel), and group 5 (CLI + EPCSW-Mel + SNAP), and ischemia-involved quadriceps were harvested by day 14.
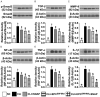
Results: An in vitro study showed that at time points of 24/48/72 h, the cell viability/protein expression of endothelial nitric oxide synthase (eNOS)/and cellular expression of nitric oxide (NO) were highest in EPCs, lowest in EPCs + menadione, and much higher in EPCSW-Mel + Mena than in EPCs + Mena + Mel. Protein levels of oxidative-stress (NOX-1/NOX-2/oxidized protein)/early (AN-V+/PI-)/late (AN-V+/PI+) apoptosis and total intracellular/mitochondrial reactive oxygen species ROS exhibited an antithetical trend of cell viability among the groups (all P<0.0001). Matrigel assay of angiogenesis/positively-stained NO cells showed that they were much higher in EPCs + SNAP than in EPCs only (all P<0.0001). Ex vivo angiogenesis/arterial relaxation of carotid-artery rings were highest in left-common-carotid-artery (LCCA) + SNAP, lowest in LCCA + Mena, and notably higher in LCCA than in LCCA + Mena + SNAP (all P<0.0001). Laser Doppler showed ischemic to normal-blood-flow (INBF) ratio was highest in group 1, lowest in group 2, and it progressively increased from groups 3 to 5 (all P<0.0001). The protein levels of oxidative-stress (NOX-1/NOX-4/oxidized protein)/apoptotic [cleaved-caspase-3/cleaved apoptosis/mitochondrial-damage (cytosolic-cytochrome-C/p-DRP-1)]/fibrotic (Smad3/TGF-β)/inflammatory (MMP-9/IL-1β/TNF-α/NF-κB) biomarkers, exhibited an opposite trend, whereas the protein level of endothelial-cell surface markers (CD31/vWF/eNOS) and number of small vessels exhibited an identical pattern of INBF ratio among the groups (all P<0.0001).
Conclusions: Combined EPCSW-Mel and SNAP therapy offered a synergic effect toward rescuing from CLI.
Keywords: Critical limb ischemia; angiogenesis; endothelial progenitor cells; melatonin; nitric oxide donor; shock wave.
AJTR Copyright © 2024.
Conflict of interest statement
None.
Figures









References
-
- Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. - PubMed
-
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. - PubMed
-
- Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. - PubMed
-
- Vemulapalli S, Patel MR, Jones WS. Limb ischemia: cardiovascular diagnosis and management from head to toe. Curr Cardiol Rep. 2015;17:611. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
