This is a preprint.
Lipid Trajectories Improve Risk Models for Alzheimer's Disease and Mild Cognitive Impairment
- PMID: 39399044
- PMCID: PMC11469357
- DOI: 10.1101/2024.09.27.24314494
Lipid Trajectories Improve Risk Models for Alzheimer's Disease and Mild Cognitive Impairment
Update in
-
Lipid trajectories improve risk models for Alzheimer's disease and mild cognitive impairment.J Lipid Res. 2025 Jan;66(1):100714. doi: 10.1016/j.jlr.2024.100714. Epub 2024 Nov 23. J Lipid Res. 2025. PMID: 39586400 Free PMC article.
Abstract
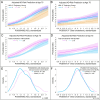
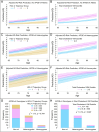
To assess the relationship between lipids and cognitive dysfunction, we retrospectively analyzed blood-lipid levels in clinically well-characterized individuals with stable mild cognitive impairment (MCI) or Alzheimer's disease (AD) over the decade prior to first cognitive symptoms. In this case/control cohort study, AD and MCI cases were diagnosed using DSM-IV criteria; MCI cases had not progressed to dementia for ≥5 years; and controls were propensity matched to cases at age of symptom onset (MCI: 116 cases, 435 controls; AD: 215 cases, 483 controls). Participants were grouped based on longitudinal trajectories and quintile of variability independent of the mean (VIM) for total cholesterol, HDL-C, LDL-C, non-HDL-C and ln(triglycerides). Models for the risk of cognitive dysfunction evaluated trajectory and VIM groups, APOE genotype, polygenic risk scores (PRS) for AD and lipid levels, age, comorbidities, and longitudinal correlates of blood-lipid concentrations. Lower HDL-C trajectories (OR = 3.8, 95% CI = 1.3-11.3) and the lowest VIM quintile of non-HDL-C (OR = 2.2, 95% CI = 1.3-3.0) were associated with higher MCI risk. Lower HDL-C trajectories (OR = 3.0, 95% CI = 1.6-5.7) and the lowest VIM quintile of total cholesterol (OR = 2.4, 95% CI = 1.5-3.9) were associated with higher AD risk. The inclusion of lipid-trajectory and VIM groups improved risk-model predictive performance independent of APOE genotype or PRS for AD and lipid levels. These results provide an important real-world perspective on the influence of lipid metabolism and blood-lipid levels on the development of stable MCI and AD.
Keywords: APOE; Alzheimer’s disease; HDL-C; LDL; cholesterol; group-based trajectory analysis; lipids; polygenic risk score; triglycerides; variability independent of the mean.
Figures







Similar articles
-
Lipid trajectories improve risk models for Alzheimer's disease and mild cognitive impairment.J Lipid Res. 2025 Jan;66(1):100714. doi: 10.1016/j.jlr.2024.100714. Epub 2024 Nov 23. J Lipid Res. 2025. PMID: 39586400 Free PMC article.
-
Elevated serum TC and LDL-C levels in Alzheimer's disease and mild cognitive impairment: A meta-analysis study.Brain Res. 2020 Jan 15;1727:146554. doi: 10.1016/j.brainres.2019.146554. Epub 2019 Nov 23. Brain Res. 2020. PMID: 31765631
-
Predictability of polygenic risk score for progression to dementia and its interaction with APOE ε4 in mild cognitive impairment.Transl Neurodegener. 2021 Aug 31;10(1):32. doi: 10.1186/s40035-021-00259-w. Transl Neurodegener. 2021. PMID: 34465370 Free PMC article.
-
Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment.Cochrane Database Syst Rev. 2020 Mar 2;3(3):CD009628. doi: 10.1002/14651858.CD009628.pub2. Cochrane Database Syst Rev. 2020. PMID: 32119112 Free PMC article.
-
Updating the Evidence on the Association between Serum Cholesterol and Risk of Late-Life Dementia: Review and Meta-Analysis.J Alzheimers Dis. 2017;56(1):215-228. doi: 10.3233/JAD-160826. J Alzheimers Dis. 2017. PMID: 27911314 Free PMC article. Review.
References
-
- Cesari M, Grande G, Canevelli M, Vanacore N, Bruno G, Quarchioni E, et al. Spontaneous Reversion of Mild Cognitive Impairment to Normal Cognition: A Systematic Review of Literature and Meta-Analysis. 2016. Jan 1; - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
