This is a preprint.
A novel alpha-synuclein G14R missense variant is associated with atypical neuropathological features
- PMID: 39399048
- PMCID: PMC11469355
- DOI: 10.1101/2024.09.23.24313864
A novel alpha-synuclein G14R missense variant is associated with atypical neuropathological features
Update in
-
A novel alpha-synuclein G14R missense variant is associated with atypical neuropathological features.Mol Neurodegener. 2025 Sep 26;20(1):98. doi: 10.1186/s13024-025-00889-y. Mol Neurodegener. 2025. PMID: 41013605 Free PMC article.
Abstract
Background: Parkinson's disease (PD) affects millions of people worldwide, but only 5-10% of patients suffer from a monogenic form of the disease with Mendelian inheritance. SNCA, the gene encoding for the protein alpha-synuclein (aSyn), was the first to be associated with familial forms of PD and, since then, several missense variants and multiplications of the SNCA gene have been established as rare causes of autosomal dominant forms of PD.
Aim and methods: A patient carrying aSyn missense mutation and his family members were studied. We present the clinical features, genetic testing - whole exome sequencing (WES), and neuropathological findings. The functional consequences of this aSyn variant were extensively investigated using biochemical, biophysical, and cellular assays.
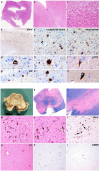
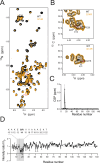
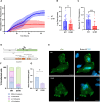
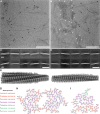
Results: The patient exhibited a complex neurodegenerative disease that included generalized myocloni, bradykinesia, dystonia of the left arm and apraxia. WES identified a novel heterozygous SNCA variant (cDNA 40G>A; protein G14R). Neuropathological examination showed extensive atypical aSyn pathology with frontotemporal lobar degeneration (FTLD) and nigral degeneration pattern with abundant ring-like neuronal inclusions, and few oligodendroglial inclusions. Sanger sequencing confirmed the SNCA variant in the healthy, elderly parent of the patient patient suggesting incomplete penetrance. NMR studies suggest that the G14R mutation induces a local structural alteration in aSyn, and lower thioflavin T binding in in vitro fibrillization assays. Interestingly, the G14R aSyn fibers display different fibrillar morphologies as revealed by cryo-electron microscopy. Cellular studies of the G14R variant revealed increased inclusion formation, enhanced membrane association, and impaired dynamic reversibility of serine-129 phosphorylation.
Summary: The atypical neuropathological features observed, which are reminiscent of those observed for the G51D aSyn variant, suggest a causal role of the SNCA variant with a distinct clinical and pathological phenotype, which is further supported by the properties of the mutant aSyn, compatible with the strain hypothesis of proteinopathies.
Keywords: Parkinson’s disease; aggregation; alpha-synuclein; bradykinesia; dystonia.
Conflict of interest statement
Conflict of interests The authors have no relevant conflicts of interest to disclose related to the article.
Figures


References
-
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease Science 1997; 276: 2045–47 - PubMed
-
- Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M α-synuclein in Lewy bodies Nature 1997; 388: 839–40 - PubMed
-
- Golbe LI, Di Iorio G, Sanges G, et al. Clinical genetic analysis of Parkinson’s disease in the Contursi kindred Ann Neurol 1996; 40: 767–75 - PubMed
-
- Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, et al. Alpha-synuclein pH50Q, a novel pathogenic mutation for Parkinson’s disease Movement Disorders 2013; 28: 811–13 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
