New anti-ovarian cancer quinolone derivatives acting by modulating microRNA processing machinery
- PMID: 39399313
- PMCID: PMC11467779
- DOI: 10.1039/d4md00649f
New anti-ovarian cancer quinolone derivatives acting by modulating microRNA processing machinery
Abstract
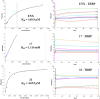
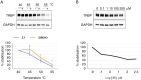
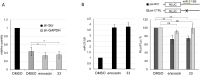
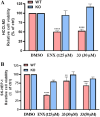
MicroRNAs (miRNAs) play a crucial role in ovarian cancer (OC) pathogenesis and miRNA processing can be the object of pharmacological intervention. By exploiting our in-house quinolone library, we combined a cell-based screening with medicinal chemistry efforts, ultimately leading to derivative 33 with anti-OC activity against distinct cell lines (GI50 values 13.52-31.04 μM) and CC50 Wi-38 = 142.9 μM. Compound 33 retained anticancer activity against additional cancer cells and demonstrated a synergistic effect with cisplatin against cisplatin-resistant A2780 cells. Compound 33 bound TRBP by SPR (K D = 4.09 μM) and thermal shift assays and its activity was TRBP-dependent, leading to modulation of siRNA and miRNA maturation. Derivative 33 exhibited augmented potency against OC cells and a stronger binding affinity for TRBP compared to enoxacin, the sole quinolone identified as a modulator of miRNA maturation. Consequently, 33 represents a promising template for developing novel anti-OC agents with a distinctive mechanism of action.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

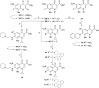
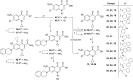
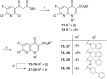
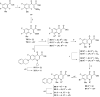
References
-
- Arora T., Mullangi S. and Lekkala M. R., in Ovarian Cancer, StatPearls Publishing, Treasure Island (FL), 2023 - PubMed
LinkOut - more resources
Full Text Sources

