Simulation-based assessment of zwitterionic pendant group variations on the hemocompatibility of polyethersulfone membranes
- PMID: 39399384
- PMCID: PMC11412084
- DOI: 10.1186/s42252-024-00062-6
Simulation-based assessment of zwitterionic pendant group variations on the hemocompatibility of polyethersulfone membranes
Abstract
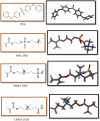
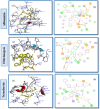
In the realm of hemodialysis, Polyethersulfone (PES) membranes dominate due to their exceptional stability and mechanical properties, capturing 93% of the market. Despite their widespread usage, the hydrophobic nature of PES introduces complications in hemodialysis, potentially leading to severe adverse reactions in patients with end-stage renal disease (ESRD) through protein fouling. Addressing this issue, our study focused on enhancing hemocompatibility by modifying PES surfaces with zwitterionic materials, known for their hydrophilicity and biological membrane compatibility. We investigated the functionalization of PES membranes utilizing various zwitterions in different ratios. Utilizing molecular docking, we examined the interactions of three zwitterionic ligands-carboxybetaine methacrylate (CBMA), sulfobetaine methacrylate (SBMA), and (2-(methacryloyloxy)ethyl) phosphorylcholine (MPC)-with human serum proteins. Our analysis revealed that a 1:1 ratio of phosphobetaine and sulfobetaine exhibits the lowest affinity energy towards serum proteins, denoting an optimal hemocompatibility without the limitations associated with increased zwitterion ratios. This pivotal finding offers a new pathway for developing more efficient and safer hemodialysis membranes, promising improved care for ESRD patients.
Supplementary information: The online version contains supplementary material available at 10.1186/s42252-024-00062-6.
Keywords: Affinity energy; Carboxybetaine; Hemocompatibility; Molecular docking; Pendant groups; Phosphobetaine; Protein-ligand interaction; Sulfobetaine.
© The Author(s) 2024.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.Declaration of competing interestThe authors have no conflict of interest to declare.
Figures








Similar articles
-
Influence of UV-irradiation intensity and exposure duration on the hemobiocompatibility enhancement of a novel synthesized phosphobetaine zwitterions polyethersulfone clinical hemodialysis membranes.J Biomed Mater Res B Appl Biomater. 2022 Mar;110(3):573-586. doi: 10.1002/jbm.b.34936. Epub 2021 Sep 12. J Biomed Mater Res B Appl Biomater. 2022. PMID: 34510718
-
Aminolysis-Based Zwitterionic Immobilization on Polyethersulfone Membranes for Enhanced Hemocompatibility: Experimental, Computational, and Ex Vivo Investigations.Biomimetics (Basel). 2024 May 27;9(6):320. doi: 10.3390/biomimetics9060320. Biomimetics (Basel). 2024. PMID: 38921200 Free PMC article.
-
Biocompatibility enhancement of hemodialysis membranes using a novel zwitterionic copolymer: Experimental, in situ synchrotron imaging, molecular docking, and clinical inflammatory biomarkers investigations.Mater Sci Eng C Mater Biol Appl. 2020 Dec;117:111301. doi: 10.1016/j.msec.2020.111301. Epub 2020 Jul 25. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32919662
-
Overview of PES biocompatible/hemodialysis membranes: PES-blood interactions and modification techniques.Mater Sci Eng C Mater Biol Appl. 2015 Nov 1;56:574-92. doi: 10.1016/j.msec.2015.06.035. Epub 2015 Jun 19. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 26249629 Review.
-
Functional Zwitterionic Polyurethanes: State-of-the-Art Review.Macromol Rapid Commun. 2024 Mar;45(5):e2300606. doi: 10.1002/marc.202300606. Epub 2023 Dec 20. Macromol Rapid Commun. 2024. PMID: 38087799 Review.
Cited by
-
Machine learning models for predicting interaction affinity energy between human serum proteins and hemodialysis membrane materials.Sci Rep. 2025 Jan 28;15(1):3474. doi: 10.1038/s41598-024-83674-z. Sci Rep. 2025. PMID: 39875505 Free PMC article.
References
-
- A. Abdelrasoul, A. Shoker, Chem. Eng. Res. Des. 177, 615–624 (2022)
-
- X. Zheng, C. Ni, W. Xiao, G. Yu, Y. Li, Sep. Purif. Technol. 298, 121464 (2022)
LinkOut - more resources
Full Text Sources
