Notoginsenoside R1, a metabolite from Panax notoginseng (Burkill) F.H.Chen, stimulates insulin secretion through activation of phosphatidylinositol 3-kinase (PI3K)/Akt pathway
- PMID: 39399466
- PMCID: PMC11466869
- DOI: 10.3389/fphar.2024.1478917
Notoginsenoside R1, a metabolite from Panax notoginseng (Burkill) F.H.Chen, stimulates insulin secretion through activation of phosphatidylinositol 3-kinase (PI3K)/Akt pathway
Abstract
Background: For ages, botanical medicine has been used in the treatment of diabetes mellitus (DM). Notoginsenoside R1 (NGR1), a Panax notoginseng (Burkill) F.H.Chen metabolite, has been documented to possess antidiabetic action in vivo. However, its precise molecular mechanism of action is not clear.
Objectives: We evaluated NGR1's effects on blood glucose in vivo and then evaluated in vitro whether NGR1 has effects on insulin secretion and the probable molecular pathways involved in NGR1-induced insulin secretion.
Methods: Diabetes was induced in mice by streptozotocin. Glucose tolerance test was performed before and after NGR1 was administered intraperitoneally to diabetic animals for 4 weeks. Static and perifusion experiments were performed using isolated female BALB/c mouse islets. Preproinsulin (Ins) mRNA expression was measured using q-PCR. Protein expression of PI3K/Akt pathway was assessed using the fully automated Wes™ capillary-based protein electrophoresis.
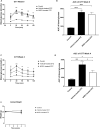
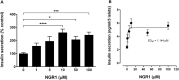
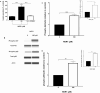
Results: Treatment of diabetic mice with NGR1 improved their glucose intolerance. In vitro, NGR1 increased insulin secretion in a concentration-dependent manner. NGR1 initiated the secretion of insulin at 2 mM glucose and augmented glucose-stimulated insulin secretion which was sustained throughout NGR1 perifusion. NGR1-induced insulin secretion was not altered by a voltage gated calcium channel blocker or protein kinase A inhibitor. NGR1 did not significantly modulate Ins mRNA expression. However, NGR1 significantly increased the levels of phospho-Akt and phopho-p-85.
Conclusion: In conclusion, this study has shown that NGR1 ameliorates hyperglycemia in diabetic mice. NGR1 has a direct insulin secretagogue activity on mouse islets, stimulates insulin secretion at both basal and postprandial glucose concentrations, and activates PI3K/Akt pathway to induce insulin secretion. These results suggest that NGR1 may provide an alternative therapy to manage DM.
Keywords: Panax notoginseng; diabetes; insulin secretion; mouse islets; plant extract.
Copyright © 2024 Al-Romaiyan, Barakat, Marafie and Masocha.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Panax notoginseng saponins provide neuroprotection by regulating NgR1/RhoA/ROCK2 pathway expression, in vitro and in vivo.J Ethnopharmacol. 2016 Aug 22;190:301-12. doi: 10.1016/j.jep.2016.06.017. Epub 2016 Jun 8. J Ethnopharmacol. 2016. PMID: 27288754
-
Notoginsenoside R1 Ameliorates Cardiac Lipotoxicity Through AMPK Signaling Pathway.Front Pharmacol. 2022 Mar 17;13:864326. doi: 10.3389/fphar.2022.864326. eCollection 2022. Front Pharmacol. 2022. PMID: 35370720 Free PMC article.
-
Commiphora myrrha stimulates insulin secretion from β-cells through activation of atypical protein kinase C and mitogen-activated protein kinase.J Ethnopharmacol. 2023 Feb 10;302(Pt B):115937. doi: 10.1016/j.jep.2022.115937. Epub 2022 Nov 19. J Ethnopharmacol. 2023. PMID: 36410575
-
Notoginsenoside R1 ameliorates diabetic encephalopathy by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation.Oncotarget. 2018 Jan 16;9(10):9344-9363. doi: 10.18632/oncotarget.24295. eCollection 2018 Feb 6. Oncotarget. 2018. PMID: 29507694 Free PMC article.
-
Pharmacological properties and mechanisms of Notoginsenoside R1 in ischemia-reperfusion injury.Chin J Traumatol. 2023 Jan;26(1):20-26. doi: 10.1016/j.cjtee.2022.06.008. Epub 2022 Jul 3. Chin J Traumatol. 2023. PMID: 35922249 Free PMC article. Review.
References
-
- Al-Romaiyan A., Masocha W., Oyedemi S., Marafie S. K., Huang G. C., Jones P. M., et al. (2023a). Commiphora myrrha stimulates insulin secretion from β-cells through activation of atypical protein kinase C and mitogen-activated protein kinase. J. Ethnopharmacol. 302, 115937. 10.1016/j.jep.2022.115937 - DOI - PubMed
LinkOut - more resources
Full Text Sources

