Immunological perspectives on atherosclerotic plaque formation and progression
- PMID: 39399488
- PMCID: PMC11466832
- DOI: 10.3389/fimmu.2024.1437821
Immunological perspectives on atherosclerotic plaque formation and progression
Abstract
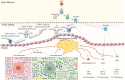
Atherosclerosis serves as the primary catalyst for numerous cardiovascular diseases. Growing evidence suggests that the immune response is involved in every stage of atherosclerotic plaque evolution. Rapid, but not specific, innate immune arms, including neutrophils, monocytes/macrophages, dendritic cells (DCs) and other innate immune cells, as well as pattern-recognition receptors and various inflammatory mediators, contribute to atherogenesis. The specific adaptive immune response, governed by T cells and B cells, antibodies, and immunomodulatory cytokines potently regulates disease activity and progression. In the inflammatory microenvironment, the heterogeneity of leukocyte subpopulations plays a very important regulatory role in plaque evolution. With advances in experimental techniques, the fine mechanisms of immune system involvement in atherosclerotic plaque evolution are becoming known. In this review, we examine the critical immune responses involved in atherosclerotic plaque evolution, in particular, looking at atherosclerosis from the perspective of evolutionary immunobiology. A comprehensive understanding of the interplay between plaque evolution and plaque immunity provides clues for strategically combating atherosclerosis.
Keywords: atherosclerosis; immune cell heterogeneity; immune response; immunotherapy; inflammatory microenvironment; plaque evolution.
Copyright © 2024 Pi, Wang, Wang, Zhang, He, Zheng, Yin, Zhao and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Innate immune system cells in atherosclerosis.Arch Med Res. 2014 Jan;45(1):1-14. doi: 10.1016/j.arcmed.2013.11.007. Epub 2013 Dec 8. Arch Med Res. 2014. PMID: 24326322 Review.
-
Atherosclerosis and immunity: A perspective.Trends Cardiovasc Med. 2019 Aug;29(6):363-371. doi: 10.1016/j.tcm.2018.09.017. Epub 2018 Sep 28. Trends Cardiovasc Med. 2019. PMID: 30292470 Review.
-
Immunobiology of Atherosclerosis: A Complex Net of Interactions.Int J Mol Sci. 2019 Oct 24;20(21):5293. doi: 10.3390/ijms20215293. Int J Mol Sci. 2019. PMID: 31653058 Free PMC article. Review.
-
Immune system and atherosclerotic disease: heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis.Circ J. 2009 Jun;73(6):994-1001. doi: 10.1253/circj.cj-09-0277. Epub 2009 May 9. Circ J. 2009. PMID: 19430164 Review.
-
Leukocyte cathepsin C deficiency attenuates atherosclerotic lesion progression by selective tuning of innate and adaptive immune responses.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):79-86. doi: 10.1161/ATVBAHA.114.304292. Epub 2014 Nov 13. Arterioscler Thromb Vasc Biol. 2015. PMID: 25395616 Free PMC article.
Cited by
-
Role of lactylation and immune infiltration in atherosclerosis: novel insights from bioinformatics analyses.Front Genet. 2025 Apr 3;16:1520325. doi: 10.3389/fgene.2025.1520325. eCollection 2025. Front Genet. 2025. PMID: 40248193 Free PMC article.
-
Colchicine for preventing stroke in patients with and without intracranial atherosclerotic stenosis: a prespecified analysis of a randomized clinical trial.EClinicalMedicine. 2025 May 19;84:103226. doi: 10.1016/j.eclinm.2025.103226. eCollection 2025 Jun. EClinicalMedicine. 2025. PMID: 40496882 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

