Lipocalin-2 drives neuropsychiatric and cutaneous disease in MRL/lpr mice
- PMID: 39399497
- PMCID: PMC11466786
- DOI: 10.3389/fimmu.2024.1466868
Lipocalin-2 drives neuropsychiatric and cutaneous disease in MRL/lpr mice
Abstract
Introduction: Approximately 20-40% of patients with systemic lupus erythematosus (SLE) experience neuropsychiatric SLE (NPSLE), which often manifests as cognitive dysfunction and depression. Currently, there are no approved treatments for NPSLE because its underlying mechanisms are unclear. Identifying relevant mediators and understanding their contribution to pathogenesis are crucial for developing targeted treatment options. Lipocalin 2 (LCN2) is a multifunctional acute-phase protein that plays important roles in immune cell differentiation, migration, and function. LCN2 has been implicated in models of neuroinflammatory disease.
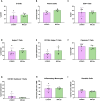
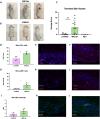
Methods: We generated an LCN2-deficient MRL/lpr mouse to evaluate the effects of LCN2 on this classic NPSLE model. To evaluate the effects of LCN2 deficiency on behavior, the mice underwent a battery of behavioral tests evaluating depression, memory, and anxiety. Flow cytometry was used to quantify immune cell populations in the brain, blood, and secondary lymphoid organs. Cutaneous disease was quantified by scoring lesional skin, and skin infiltrates were quantified through immunofluorescent staining. Systemic disease was evaluated through measuring anti-nuclear antibodies by ELISA.
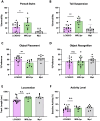
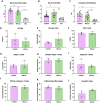
Results: In this study, we found that LCN2 deficiency significantly attenuates neuropsychiatric and cutaneous disease in MRL/lpr lupus prone mice, likely by decreasing local infiltration of immune cells into the brain and skin and reducing astrocyte activation in the hippocampus. Anti-nuclear antibodies and kidney disease were not affected by LCN2.
Discussion: As there was no effect on systemic disease, our results suggest that the inflammatory effects of LCN2 were localized to the skin and brain in this model. This study further establishes LCN2 as a potential target to ameliorate organ injury in SLE, including neuropsychiatric and cutaneous disease.
Keywords: MRL/lpr; cutaneous systemic lupus erythematosus; lipocalin-2; neuropsychiatric lupus; systemic lupus erythematosus.
Copyright © 2024 Garcia, Mike, Zhang, Cuda and Putterman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

