Laboratory considerations in the assessment of 25-hydroxyvitamin D in pregnant women by automated immunoassays
- PMID: 39399546
- PMCID: PMC11470585
- DOI: 10.1016/j.plabm.2024.e00430
Laboratory considerations in the assessment of 25-hydroxyvitamin D in pregnant women by automated immunoassays
Abstract
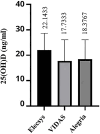
Background: Because of the pathophysiological role of vitamin D in health, there is an increased interest to check the clinical status of this vitamin. Immunochemical assays are commonly employed to determine 25-hydroxyvitamin D (25 (OH) D) in clinical laboratories and its testing could be influenced by pre-analytic and analytic issues. The aim of this study was to compare the 25(OH)D results obtained from three commonly used immunoassays in pregnant women to check a possible discrepancy between tests.
Material and methods: A group of 50 pregnant women who were in their third trimester were included in this study. The quantification of serum vitamin D was performed utilizing three immunochemistry-based assays including Elecsys, VIDAS and Alegria. We also involved 21 non-pregnant volunteers to clinically assess the vitamin D status in this group of people.
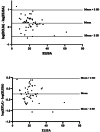
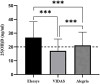
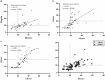
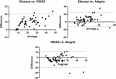
Results: Our findings revealed a significant inconsistency between the obtained results from three assays for serum 25(OH)D. The 25(OH)D showed higher values when measured by the Elecsys assay while the VIDAS assay had lower values compared to the other immunoassays. More notably, the 25(OH)D testing in non-pregnant subjects showed consistent results in all three immunoassays.
Conclusions: The results of the 25(OH)D measurements in pregnant women should be interpreted carefully due to a great inaccuracy in immunoassay testing. There is no such disagreement in non-pregnant people. Standardization of vitamin D testing in various settings is a crucial matter for clinical laboratories.
Keywords: 25-Hydroxyvitamin D; Analytical method; Immunoassay; Inconsistency; Pregnant women.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Analytical and clinical evaluation of the new Fujirebio Lumipulse®G non-competitive assay for 25(OH)-vitamin D and three immunoassays for 25(OH)D in healthy subjects, osteoporotic patients, third trimester pregnant women, healthy African subjects, hemodialyzed and intensive care patients.Clin Chem Lab Med. 2016 Aug 1;54(8):1347-55. doi: 10.1515/cclm-2015-0923. Clin Chem Lab Med. 2016. PMID: 26741345
-
Improved Recognition of 25-Hydroxyvitamin D2 by 2 Automated Immunoassays.J Appl Lab Med. 2020 Nov 1;5(6):1287-1295. doi: 10.1093/jalm/jfaa070. J Appl Lab Med. 2020. PMID: 32529210
-
Evaluation of the new restandardized 25-hydroxyvitamin D assay on the iSYS platform.Clin Biochem. 2018 Feb;52:156-160. doi: 10.1016/j.clinbiochem.2017.11.011. Epub 2017 Nov 22. Clin Biochem. 2018. PMID: 29174995
-
25-Hydroxyvitamin D Testing: Immunoassays Versus Tandem Mass Spectrometry.Clin Lab Med. 2018 Sep;38(3):439-453. doi: 10.1016/j.cll.2018.05.007. Epub 2018 Jul 20. Clin Lab Med. 2018. PMID: 30115390 Review.
-
Analytical methods for 25-hydroxyvitamin D: advantages and limitations of the existing assays.J Nutr Biochem. 2022 Nov;109:109123. doi: 10.1016/j.jnutbio.2022.109123. Epub 2022 Aug 4. J Nutr Biochem. 2022. PMID: 35934272 Review.
References
-
- Chang S.W., Lee H.C. Vitamin D and health - the missing vitamin in humans. Pediatr Neonatol. 2019;60(3):237–244. - PubMed
-
- Zerwekh J.E. The measurement of vitamin D: analytical aspects. Ann. Clin. Biochem. 2004;41(Pt 4):272–281. - PubMed
-
- Vogiatzi M.G., Jacobson-Dickman E., DeBoer M.D. Drugs, Therapeutics Committee of the Pediatric Endocrine S. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J. Clin. Endocrinol. Metab. 2014;99(4):1132–1141. - PubMed
-
- Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. - PubMed
LinkOut - more resources
Full Text Sources

