This is a preprint.
Nageotte nodules in human DRG reveal neurodegeneration in painful diabetic neuropathy
- PMID: 39399674
- PMCID: PMC11469377
- DOI: 10.21203/rs.3.rs-5006011/v1
Nageotte nodules in human DRG reveal neurodegeneration in painful diabetic neuropathy
Update in
-
Nageotte nodules in human dorsal root ganglia reveal neurodegeneration in diabetic peripheral neuropathy.Nat Commun. 2025 May 5;16(1):4168. doi: 10.1038/s41467-025-59538-z. Nat Commun. 2025. PMID: 40325011 Free PMC article.
Abstract
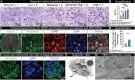
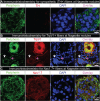
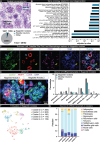
Diabetic neuropathy is frequently accompanied by pain and loss of sensation attributed to axonal dieback. We recovered dorsal root ganglia (DRGs) from 90 organ donors, 19 of whom had medical indices for diabetic painful neuropathy (DPN). Nageotte nodules, dead sensory neurons engulfed by non-neuronal cells, were abundant in DPN DRGs and accounted for 25% of all neurons. Peripherin-and Nav1.7-positive dystrophic axons invaded Nageotte nodules, forming small neuroma-like structures. Using histology and spatial sequencing, we demonstrate that Nageotte nodules are mainly composed of satellite glia and non-myelinating Schwann cells that express SPP1 and are intertwined with sprouting sensory axons originating from neighboring neurons. Our findings solve a 100-year mystery of the nature of Nageotte nodules linking these pathological structures to pain and sensory loss in DPN.
Conflict of interest statement
Declarations Competing interests: TJP is a co-founder of 4E Therapeutics. The authors declare no other competing interests related to this work.
Figures


References
-
- Nageotte J. L’organisation de la matiere dans ses rapports avec la vie: etudes d’anatomie generale et de morphologie experimentale sur le tissu conjonctif et le nerf. (Alcan F., 1922).
-
- Lakritz J. R. et al. alpha4-Integrin Antibody Treatment Blocks Monocyte/Macrophage Traffic to, Vascular Cell Adhesion Molecule-1 Expression in, and Pathology of the Dorsal Root Ganglia in an SIV Macaque Model of HIV-Peripheral Neuropathy. Am J Pathol 186, 1754–1761, doi:10.1016/j.ajpath.2016.03.007 (2016). - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

