Development and Content Validation of the Patient's Qualitative Assessment of Treatment - Real-World (PQAT-RW): An Instrument to Evaluate Benefits and Disadvantages of Treatments in Real-World Settings
- PMID: 39399824
- PMCID: PMC11468370
- DOI: 10.2147/PROM.S468623
Development and Content Validation of the Patient's Qualitative Assessment of Treatment - Real-World (PQAT-RW): An Instrument to Evaluate Benefits and Disadvantages of Treatments in Real-World Settings
Abstract
Purpose: Quantifying patient-perceived benefits and disadvantages of treatments in a real-world setting is increasingly important in healthcare decision-making. The Patient's Qualitative Assessment of Treatment (PQAT) assesses patient-perceived benefits and disadvantages of treatment, and associated trade-offs potentially influencing patients' willingness to continue treatment. It has then been modified to capture patients' perceived magnitude of benefits and disadvantages of treatment quantitatively, as well as qualitatively (PQATv2). However, the PQAT and the PQATv2 were designed for use and validated in a clinical trial setting. The objective of this study was to adapt and test the content validity of a version of the PQATv2 for use in real-world settings (PQAT-RW).
Patients and methods: The PQATv2 was adapted for use in real-world settings (PQAT-RW), and its content was validated in 16 patients with varied chronic medical conditions and medication regimens via semi-structured qualitative interviews.
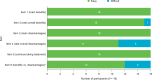
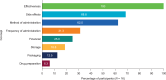
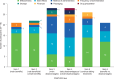
Results: All participants reported that the PQAT-RW was "easy to understand". The majority (n = 11/16) reported that the items covered all important aspects of their treatment experience, and that no items needed to be removed or added to the instrument. Analysis of free-text responses identified eight global concepts considered by participants when evaluating the benefits and disadvantages of treatment: treatment effectiveness, side effects and method of administration were most frequently considered (as both benefits and disadvantages), followed by frequency of administration, financial considerations, storage, packaging and drug preparation.
Conclusion: The results of this study support the content validity of the PQAT-RW. They also demonstrate that using qualitative responses to contextualize quantitative responses provides unique insight into diverse and individualized patient-perceived benefits and disadvantages, and their relative importance, in real-world settings.
Keywords: patient-reported outcome measure; qualitative research; real-world evidence; treatment experience.
© 2024 Roborel de Climens et al.
Conflict of interest statement
Adam Gater is employed by Adelphi Values, which has received funding from Sanofi to analyse data reported in this paper. Amy Findley was employed by Adelphi Values at the time of this study and is now employed by Novo Nordisk. Denise P. Bury was a paid employee of Sanofi at the time of this study and may hold shares and/or stock options in the company and is now an employee at Novartis. Keri J. S. Brady was a paid employee of Sanofi at the time of this study and may hold shares and/or stock options in the company. Keri is now an employee at AbbVie. Aude Roborel de Climens was a paid employee and stockholder of Sanofi at the time of this study. Matthew Reaney was previously an employee at Sanofi, which holds the copyright and distribution rights to the PQATv2 and PQAT-RW. Aude and Matthew are now employees at IQVIA, a healthcare research organization that conducts research on behalf of Sanofi and other pharmaceutical companies. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Development and First Use of the Patient's Qualitative Assessment of Treatment (PQAT) Questionnaire in Type 2 Diabetes Mellitus to Explore Individualised Benefit-Harm of Drugs Received During Clinical Studies.Drug Saf. 2020 Feb;43(2):119-134. doi: 10.1007/s40264-019-00877-4. Drug Saf. 2020. PMID: 31679129 Free PMC article.
-
Development of a New Tool for Evaluating the Benefit of Preventive Treatments for Migraine on Functional Outcomes - The Migraine Functional Impact Questionnaire (MFIQ).Headache. 2018 Nov;58(10):1612-1628. doi: 10.1111/head.13420. Epub 2018 Oct 30. Headache. 2018. PMID: 30374958 Free PMC article.
-
Bisphosphonate alternative regimens for the prevention of osteoporotic fragility fractures: BLAST-OFF, a mixed-methods study.Health Technol Assess. 2024 Apr;28(21):1-169. doi: 10.3310/WYPF0472. Health Technol Assess. 2024. PMID: 38634483 Free PMC article.
-
Using real-world data for coverage and payment decisions: the ISPOR Real-World Data Task Force report.Value Health. 2007 Sep-Oct;10(5):326-35. doi: 10.1111/j.1524-4733.2007.00186.x. Value Health. 2007. PMID: 17888097
-
A qualitative systematic review of internal and external influences on shared decision-making in all health care settings.JBI Libr Syst Rev. 2012;10(58):4633-4646. doi: 10.11124/jbisrir-2012-432. JBI Libr Syst Rev. 2012. PMID: 27820528
Cited by
-
Study Protocol: Investigating the Effects of Transcranial Pulse Stimulation in Parkinson's Disease.Bioengineering (Basel). 2025 Jul 17;12(7):773. doi: 10.3390/bioengineering12070773. Bioengineering (Basel). 2025. PMID: 40722464 Free PMC article.
References
-
- Food and Drug Administration. The voice of the patient: a series of reports from FDA’s patient-focused drug development initiative; 2017. Available from: https://www.fda.gov/ForIndustry/UserFees/PrescriptionDrugUserFee/ucm3683.... Accessed June, 2021.
-
- Food and Drug Administration. Factors to consider when making benefit-risk determinations in medical device premarket approval and De Novo classifications; 2019. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed September 2021.
-
- Food and Drug Administration. Structured approach to benefit-risk assessment in drug regulatory decision-making; 2013. Available from: https://www.fda.gov/files/about%20fda/published/PDUFA-V-Implementation-P.... Accessed September, 2021.
LinkOut - more resources
Full Text Sources