Atogepant for migraine prevention: a meta-analysis of safety and efficacy in adults
- PMID: 39399876
- PMCID: PMC11466836
- DOI: 10.3389/fneur.2024.1468961
Atogepant for migraine prevention: a meta-analysis of safety and efficacy in adults
Erratum in
-
Corrigendum: Atogepant for migraine prevention: a meta-analysis of safety and efficacy in adults.Front Neurol. 2025 Apr 10;16:1577914. doi: 10.3389/fneur.2025.1577914. eCollection 2025. Front Neurol. 2025. PMID: 40291850 Free PMC article.
Abstract
Background: Migraine is a neurological condition marked by frequent headaches, which tends to be accompanied by nausea and vomiting in severe instances. Injectable therapies for migraine, such as monoclonal antibodies that target calcitonin gene-related peptide (CGRP), have proven to be effective and safe. While various oral drugs are available, none have been developed for migraines. Patients prefer oral therapies because they are easier to use, making atogepant, an orally accessible small-molecule CGRP receptor antagonist, a possible alternative.
Objectives: This systematic review and meta-analysis compared the safety and effectiveness of atogepant with placebo in treating migraine.
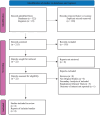
Methods: Adhering to the PRISMA guidelines, we meticulously gathered randomized controlled trials (RCTs) from databases including the Cochrane Library, PubMed, Science Direct, and ClinicalTrials.gov. Studies comparing atogepant with placebo and reporting monthly migraine days (MMDs) as the primary outcome along with secondary outcomes such as monthly headache days and acute medication use days were included. Two independent reviewers conducted the data extraction and quality assessment. Statistical analyses were carried out using RevMan, utilizing risk ratios for dichotomous outcomes and mean differences for continuous outcomes, and a random-effects model.
Results: Our primary outcome was the change in MMDs over 12 weeks, which showed a significant reduction with atogepant at dosages of 10, 30, and 60 mg. Secondary outcomes, such as monthly headache days, proportion of patients achieving a ≥ 50% reduction in MMDs, acute medication use days, and patient-reported outcomes, consistently showed that atogepant outperformed placebo, highlighting its effectiveness in reducing the migraine burden.
Conclusion: Higher doses of atogepant are more effective in lowering migraine and headache-related days and increasing quality of life metrics. However, this is accompanied by an increased incidence of adverse events, suggesting the need for careful dose optimization to balance the benefits and risks.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=563395. Unique Identifier: CRD42024563395.
Keywords: CGRP; atogepant; headache; meta-analysis; migraine.
Copyright © 2024 Raja, Asim, Shuja, Raja, Muhammad, Bajaj, Ansari, Ali, Magsi, Faridi, Sheikh, Imran, Ahmed and Asghar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
-
- Buse DC, Reed ML, Fanning KM, Bostic RC, Lipton RB. Demographics, headache features, and comorbidity profiles in relation to headache frequency in people with migraine: results of the American migraine prevalence and prevention (AMPP) study. Headache. (2020) 60:2340–56. doi: 10.1111/head.13966, PMID: - DOI - PubMed
-
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. (1990) 28:183–7. - PubMed
-
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the quality standards Subcommittee of the American Academy of neurology and the American headache society. Neurol Int. (2012) 78:1337–45. doi: 10.1212/WNL.0b013e3182535d20 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

