Comprehensive vs. standard remote monitoring of cardiac resynchronization devices in heart failure patients: results of the ECOST-CRT study
- PMID: 39400005
- PMCID: PMC11472153
- DOI: 10.1093/europace/euae233
Comprehensive vs. standard remote monitoring of cardiac resynchronization devices in heart failure patients: results of the ECOST-CRT study
Abstract
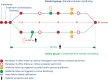
Aims: Integrating remote monitoring (RM) into existing healthcare practice for heart failure (HF) patients to improve clinical outcome remains challenging. The ECOST-CRT study compared the clinical outcome of a comprehensive RM scheme including a patient questionnaire capturing signs and symptoms of HF and notifications for HF specific parameters to traditional RM in patients with cardiac resynchronization therapy (CRT) devices.
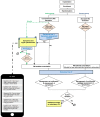
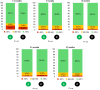
Methods and results: Patients were randomized 1:1 to standard daily RM (notification for technical parameters and ventricular arrhythmias; control group) or comprehensive RM (adding a monthly symptom questionnaire and notifications for biventricular pacing, premature ventricular contraction, atrial arrhythmias; active group). The primary endpoint was all-cause mortality or hospitalization for worsening HF (WHF). Six hundred fifty-two patients (70.4 ± 10.3 years, 73% men, left ventricular ejection fraction 29.1 ± 7.6%, 68% CRT-Defibrillators, 32% CRT-Pacemakers) were enrolled. The COVID-19 pandemic caused an early termination of the study, so the mean follow-up duration was 18 ± 8 months. No statistically significant difference in the primary endpoint was found between the groups [59 (18.3%) control vs. 77 (23.3%) active group; log-rank test P = 0.13]. Among the secondary endpoints, the MLHF questionnaire showed a larger share of patients with improvement of quality of life compared to baseline in the active group (78%) vs. control (61%; P = 0.03).
Conclusion: The study does not support the notion that comprehensive RM, when compared to standard RM, in HF patients with CRT improves the clinical outcome of all-cause mortality or WHF hospitalizations. However, this study was underpowered due to an early termination and further trials are required.
Registration: Clinical Trials.gov Identifier: NCT03012490.
Keywords: Cardiac resynchronization therapy; Heart failure; Remote monitoring.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: C. Klein received consulting fees from Boston Scientific. L.G.-M. received consulting fees from Boston Scientific and Biotronik, and has grant support from Abbott, Medtronic, Phenix Cardiologie, Microport, and Zoll. A.L. is minor part-time employee of Biotronik France, has participated on a Data Safety Monitoring Board for Biotronik, has stock options from Implicity, and has received support for attending meetings from Boston Scientific France. P.d.G. received consulting fees from Astra Zeneca, Servier, Boehringer Ingelheim, and Bayer, and honoraria from Novartis, Astra Zeneca, Servier, BMS, Vifor, MSD, Boehringer Ingelheim, Bayer, and Pfizer. E.M. received research grants and consulting fees from Medtronic, Abbott, Boston Scientific, Biotronik, Microport, and Zoll. F.M. received consulting fees Newcard, honoraria from Novartis, BMS/Pfizer, Vifor, and Boehringer, and has received support for attending meetings from Boehringer and BMS/Pfizer. B.D. received consulting fees from Boston Scientific, Abbott, and Microport. Y.G. has received support for attending meetings from Biotronik. J.M. received consulting fees from Biotronik, Boston Scientific, Medtronic, and Microport. C.L. received consulting fees and has grant support from Biotronik, Medtronic, and Abbott. C.B. has no conflict of interests.
Figures


References
-
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. - PubMed
-
- Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583–90. - PubMed
-
- Geller JC, Lewalter T, Bruun NE, Taborsky M, Bode F, Cosedis Nielsen J et al. Implant-based multi-parameter telemonitoring of patients with heart failure and a defibrillator with vs. without cardiac resynchronization therapy option: a subanalysis of the IN-TIME trial. Clin Res Cardiol 2019;108:1117–27. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

