Alzheimer's disease clinical variants show distinct neuroinflammatory profiles with neuropathology
- PMID: 39400356
- PMCID: PMC12465012
- DOI: 10.1111/nan.13009
Alzheimer's disease clinical variants show distinct neuroinflammatory profiles with neuropathology
Abstract
Aims: Although the neuroanatomical distribution of tau and amyloid-β is well studied in Alzheimer's disease (AD) (non)-amnestic clinical variants, that of neuroinflammation remains unexplored. We investigate the neuroanatomical distribution of activated myeloid cells, astrocytes, and complement alongside amyloid-β and phosphorylated tau in a clinically well-defined prospectively collected AD cohort.
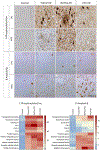
Methods: Clinical variants were diagnosed antemortem, and brain tissue was collected post-mortem. Typical AD (n = 10), behavioural/dysexecutive AD (n = 6), posterior cortical atrophy (PCA) AD (n = 3), and controls (n = 10) were neuropathologically assessed for AD neuropathology, concurrent pathology including Lewy body disease, limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC), and vascular pathology. For quantitative assessment, we analysed the corticolimbic distribution of phosphorylated tau, amyloid-β, CD68, MHC-II, C4b, and glial fibrillary acidic protein (GFAP) using digital pathology.
Results: Phosphorylated tau was distinctly distributed in each variant. In all variants, amyloid-β was neocortical-dominant, with a notable increase in the middle frontal cortex of behavioural/dysexecutive AD. Typical AD and PCA AD had no concurrent Lewy body disease, whereas three out of six cases with behavioural/dysexecutive AD did. LATE-NC stage >0 was observed in three AD cases, two typical AD (stage 1/3), and one behavioural/dysexecutive AD (stage 2/3). Vascular pathology was present in each variant. In typical AD, CD68 and MHC-II were hippocampal-dominant. In behavioural/dysexecutive AD, C4b was elevated in the middle frontal and inferior parietal cortex. In PCA AD, MHC-II was increased in the fusiform gyrus, and GFAP in parietal cortices. Correlations between AD neuropathology and neuroinflammation were distinct within variants.
Conclusions: Our data suggests that different involvement of neuroinflammation may add to clinical heterogeneity in AD, which has implications for neuroinflammation-based biomarkers and future therapeutics.
Keywords: Alzheimer's disease; amyloid; astrocytes; complement; myeloid cells; tau proteins.
© 2024 The Author(s). Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of British Neuropathological Society.
Conflict of interest statement
Dr Boon reports grants from Alzheimer Nederland during the conduct of the study; Frigerio, de Gooijer, Morrema, Bol, Galis, Dr Heymans declare no competing interests; Dr Murray reported grants from NIH/NIA, the State of Florida, and Eli Lilly and Company, and served as a paid consultant for Avid Radiopharmaceuticals during the conduct of this study; Dr Van der Lee is part of the GeneMINDS consortium, which is powered by Health~Holland, Top Sector Life Sciences & Health and receives co-financing from Vigil Neuroscience, Prevail therapeutics and Brain Research Center, all funding is paid to his institution. He was funded for this study by NWO (#733050512, PROMO-GENODE: a PROspective study of MOnoGEnic causes Of Dementia) a substantial donation by Edwin Bouw Fonds and Dioraphte and received further funding for the GeneMINDS consortium, which is powered by Health~Holland, Top Sector Life Sciences & Health. He is part of the YOD-INCLUDED project, which is funded by ZonMw (project no. 10510032120002) and is part of the Dutch Dementia Research Programme. He is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW (#73305095007), de Hersenstichting, Edwin Bouw Fonds, Gieskes-Strijbisfonds, Health~Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). Dr Van de Berg was financially supported by grants from Dutch Research council (ZonMW), Stichting Parkinson Fonds, Alzheimer association, Michael J Fox foundation, Parkinson Association and Health Holland. Dr van de Berg performed contract research for Hoffmann-La Roche, Roche Tissue Diagnostics, Crossbeta Sciences, DiscovericBio. All payments were made to the institution. Dr Van de Berg participates in the scientific advisory board of GT Gain Therapeutics SA; Dr Jonkman reports grants from the Alzheimer Association, Alzheimer Netherlands, Michael J Fox Foundation for Parkinson’s disease, Stichting Parkinson Fonds, Health Holland public-private partnership, and the Netherlands Organization for Health Research and Development. All payments were made to the institution; Prof. Dr Rozemuller reports no competing interests; Dr Bouwman performs contract research for Optina Dx and Optos, she has been an invited speaker at Roche and has been invited for expert testimony at Biogen. All funding is paid to her institution; Dr Hoozemans received grants from the Dutch Research Council (ZonMW) and, Alzheimer Netherlands, performed contract research or received grants from Merck, ONO Pharmaceuticals, Janssen Prevention Center, DiscovericBio, AxonNeurosciences, Roche, Genentech, Promis, Denali, FirstBiotherapeutics, and Ensol Biosciences. All payments were made to the institution. Dr Hoozemans participates in the scientific advisory board of Alzheimer Netherlands and is editor-in-chief for Acta Neuropathologica Communications.
Figures


References
-
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. May 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

