Revealing the different levels of action monitoring in visuomotor transformation task: Evidence from decomposition of cortical potentials
- PMID: 39400360
- PMCID: PMC11785542
- DOI: 10.1111/psyp.14708
Revealing the different levels of action monitoring in visuomotor transformation task: Evidence from decomposition of cortical potentials
Abstract
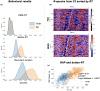
This study investigates the cortical correlates of motor response control and monitoring, using the Theory of Event Coding (TEC) as a framework to investigate signals related to low-level sensory processing of motor reafference and high-level response monitoring, including verification of response outcomes with the internal model. We used a visuomotor paradigm with two targets at different distances from the participant. For the recorded movement-related cortical potentials (MRCPs), we analyzed their different components and assessed the movement phases during which they are active. Residual iteration decomposition (RIDE) and multivariate pattern analysis (MVPA) were used for this analysis. Using RIDE, we separated MRCPs into signals related to different parallel processes of visuomotor transformation: stimulus processing (S-cluster), motor response preparation and execution (R-cluster), and intermediate processes (C-cluster). We revealed sequential activation in the R-cluster, with execution-related negative components and positive contralateral peaks reflecting reafference processing. We also identified the motor post-imperative negative variation within the R-cluster, highlighting the response outcome evaluation process included in the action file. Our findings extend the understanding of C-cluster signals, typically associated with stimulus-response mapping, by demonstrating C-activation from the preparatory stages through to response termination, highlighting its participation in action monitoring. In addition, we highlighted the ability of MVPA to identify movement-related attribute encoding: where statistical analysis showed independence of stimulus processing activity from movement distance, MVPA revealed distance-related differences in the S-cluster within a time window aligned with the lateralized readiness potential (LRP). This highlights the importance of integrating RIDE and MVPA to uncover the intricate neural dynamics of motor control, sensory integration, and response monitoring.
Keywords: P3; multivariate pattern analysis; reafferent potential; residue iteration decomposition; response monitoring; theory of event coding; visuomotor transformation.
© 2024 The Author(s). Psychophysiology published by Wiley Periodicals LLC on behalf of Society for Psychophysiological Research.
Conflict of interest statement
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Figures





References
-
- Bender, S. , Resch, F. , Klein, C. , Renner, T. , Fallgatter, A. J. , Weisbrod, M. , & Romanos, M. (2012). Influence of stimulant medication and response speed on lateralization of movement‐related potentials in attention‐deficit/hyperactivity disorder. PLoS One, 7(6), e39012. 10.1371/journal.pone.0039012 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

