Impact of TP53 Mutation Status in Elderly AML Patients When Adding All-Trans Retinoic Acid or Valproic Acid to Decitabine
- PMID: 39400388
- PMCID: PMC11707815
- DOI: 10.1111/ejh.14304
Impact of TP53 Mutation Status in Elderly AML Patients When Adding All-Trans Retinoic Acid or Valproic Acid to Decitabine
Abstract
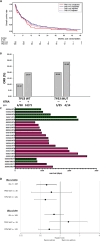
In a randomized phase II trial (AMLSG 14-09, NCT00867672) of elderly, newly diagnosed AML patients, ATRA combined with decitabine (DEC) significantly improved the overall response rate (ORR) and survival also in patients with adverse-risk genetics, without adding toxicity. We performed a post hoc analysis to determine the predictive impact of TP53 status. Despite a nominally higher ORR, the clinically meaningful survival benefit when adding ATRA to DEC was diminished, but not completely negated, in TP53-mutated patients. Indeed, 2 out of 14 TP53-mutated patients (14%) randomized to a DEC + ATRA-containing regimen lived for > 36 months. Further studies of ATRA combined with hypomethylating agents appear warranted in non-M3 AML patients ineligible for HMA/venetoclax therapy. Trial Registration: ClinicalTrials.gov identifier: NCT00867672.
Keywords: TP53; AML; ATRA; HMA; epigenetics.
© 2024 The Author(s). European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
Crysandt
Incyte: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Thol
Abbvie: Honoraria; Pfizer: Honoraria; Astellas: Honoraria; Novartis: Honoraria; BMS/Celgene: Honoraria, Research Funding; Jazz: Honoraria.
Heuser
Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tolremo: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; BergenBio: Research Funding; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer Pharma AG: Research Funding; Astellas: Research Funding.
Götze
Abbvie: Advisory Board; BMS/Celgene: Advisory Board, Research Funding.
Schlenk
Agios: Honoraria; Astellas: Honoraria, Research Funding, Speakers Bureau; Celgene: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; Abbvie: Honoraria; Hexal: Honoraria; Neovio Biotech: Honoraria; Novartis: Honoraria; Pfizer: Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Research Funding; AstraZeneca: Research Funding; Boehringer Ingelheim: Research Funding.
Salih
Synimmune GmbH: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Celgene: Honoraria; BMS: Honoraria.
Schittenhelm
Takeda: advisory board; Astellas: advisory board; BMS: advisory board; University of Tuebingen: Patents & Royalties: patent for ASPP2k.
Müller‐Tidow
Pfizer: Research Funding; Janssen: Consultancy, Research Funding; Bioline: Research Funding; Astellas: lecture fees. Germing: speakers honorarium: BMS, Novartis, Janssen: speakers honorarium; BMS, Novartis, Abbvie, Jazz: institutional research report; BMS: Advice.
Giagounidis
Novartis: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Bug: Novartis: Support of Investigator‐Initiated Trial to my Institution; Jazz, Gilead, Novartis, BMS, Pfizer: Honoraria; Jazz, Neovii: Travel Grants.
Wäsch
Amgen: Consultancy, Honoraria; Pfizer: Consultancy; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Novartis: Consultancy; BMS/Celgene: Consultancy; Gilead: Consultancy.
Döhner
Jazz Roche: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Advisory Board; Agios and Astex: Research Funding; Abbvie: Consultancy, Honoraria; Janssen: Honoraria, Advisory Board; Astellas: Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Ganser
Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria; Celgene: Honoraria.
Döhner
Astellas: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Oxford Biomedicals: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; Helsinn: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Berlin‐Chemie: Consultancy, Honoraria; Astex: Consultancy, Honoraria; Agios: Consultancy, Honoraria, Research Funding; Ulm University Hospital: Current Employment; Jazz: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria; GEMoaB: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Research Funding.
Hackanson
Roche: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Boehringer‐Ingelheim: Consultancy, Honoraria; MSD: Consultancy, Honoraria. Becker: BMS: Honoraria; Pierre Fabre Pharma: Honoraria; Servier: Honoraria; MSD: Honoraria; Novartis: Honoraria; Lilly: Honoraria; GSK: Honoraria.
Lübbert
Advisory Board: AbbVie, Astex, Otsuka, Syros; Research funding to our institution: Janssen‐Cilag; Research support with study drug: Cheplapharm (ATRA), Imago (Bomedemstat) OffLabel Disclosure: ATRA, in non‐M3 AML valproic acid, in non‐M3 AML.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

