A multiantigenic antibacterial nanovaccine utilizing hybrid membrane vesicles for combating Pseudomonas aeruginosa infections
- PMID: 39400457
- PMCID: PMC11472236
- DOI: 10.1002/jev2.12524
A multiantigenic antibacterial nanovaccine utilizing hybrid membrane vesicles for combating Pseudomonas aeruginosa infections
Abstract
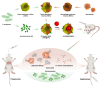
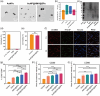
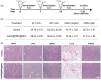
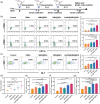
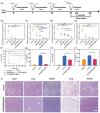
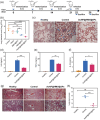
Bacterial infections, especially those caused by multidrug-resistant pathogens, pose a significant threat to public health. Vaccines are a crucial tool in fighting these infections; however, no clinically available vaccine exists for the most common bacterial infections, such as those caused by Pseudomonas aeruginosa. Herein, a multiantigenic antibacterial nanovaccine (AuNP@HMV@SPs) is reported to combat P. aeruginosa infections. This nanovaccine utilizes the hybrid membrane vesicles (HMVs) created by fusing macrophage membrane vesicles (MMVs) with bacterial outer membrane vesicles (OMVs). The HMVs mitigate the toxic effects of both OMVs and bacterial secreted toxins (SP) adsorbed on the surface of MMVs, while preserving their stimulating properties. Gold nanoparticles (AuNPs) are utilized as adjuvant to enhance immune response without comprising safety. The nanovaccine AuNP@HMV@SPs induces robust humoral and cellular immune responses, leading to destruction of bacterial cells and neutralization of their secreted toxins. In murine models of septicemia and pneumonia caused by P. aeruginosa, AuNP@HMV@SPs exhibits superior prophylactic efficacy compared to control groups including OMVs, or MMVs@SPs and HMV@SPs, achieving 100% survival in septicemia and > 99.9% reduction in lung bacterial load in pneumonia. This study highlights AuNP@HMV@SPs as a safe and effective antibacterial nanovaccine, targeting both bacteria and their secreted toxins, and offers a promising platform for developing multiantigenic antibacterial vaccines against multidrug-resistant pathogens.
Keywords: antibacterial; bacterial toxins; hybrid cell membrane; outer membrane vesicle; vaccine.
© 2024 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Chheda, D. , Shete, S. , Tanisha, T. , Bahadure, S. D. , Sampathi, S. , Junnuthula, V. , & Dyawanapelly, S. (2024). Multifaceted therapeutic applications of biomimetic nanovaccines. Drug Discovery Today, 29, 6. - PubMed
-
- Gan, Y. , Li, C. , Peng, X. , Wu, S. , Li, Y. , Tan, J. , Yang, Y. Y. , Yuan, P. , & Ding, X. (2021). Fight bacteria with bacteria: Bacterial membrane vesicles as vaccines and delivery nanocarriers against bacterial infections. Nanomed‐Nanotechnol, 35, 102398. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

