Pharmacologically increasing cGMP improves proteostasis and reduces neuropathy in mouse models of CMT1
- PMID: 39400753
- PMCID: PMC11473742
- DOI: 10.1007/s00018-024-05463-1
Pharmacologically increasing cGMP improves proteostasis and reduces neuropathy in mouse models of CMT1
Abstract
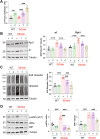
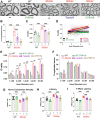
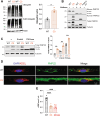
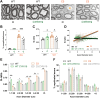
Increasing cyclic GMP activates 26S proteasomes via phosphorylation by Protein Kinase G and stimulates the intracellular degradation of misfolded proteins. Therefore, agents that raise cGMP may be useful therapeutics against neurodegenerative diseases and other diseases in which protein degradation is reduced and misfolded proteins accumulate, including Charcot Marie Tooth 1A and 1B peripheral neuropathies, for which there are no treatments. Here we increased cGMP in the S63del mouse model of CMT1B by treating for three weeks with either the phosphodiesterase 5 inhibitor tadalafil, or the brain-penetrant soluble guanylyl cyclase stimulator CYR119. Both molecules activated proteasomes in the affected peripheral nerves, reduced polyubiquitinated proteins, and improved myelin thickness and nerve conduction. CYR119 increased cGMP more than tadalafil in the peripheral nerves of S63del mice and elicited greater biochemical and functional improvements. To determine whether raising cGMP could be beneficial in other neuropathies, we first showed that polyubiquitinated proteins and the disease-causing protein accumulate in the sciatic nerves of the C3 mouse model of CMT1A. Treatment of these mice with CYR119 reduced the levels of polyubiquitinated proteins and the disease-causing protein, presumably by increasing their degradation, and improved myelination, nerve conduction, and motor coordination. Thus, pharmacological agents that increase cGMP are promising treatments for CMT1 neuropathies and may be useful against other proteotoxic and neurodegenerative diseases.
Keywords: Charcot Marie Tooth; Peripheral neuropathy; Phosphorylation; Proteasome; Protein degradation; cGMP.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
GTM and ESB were employees of Cyclerion Therapeutics and may own stock. All other authors report no conflicts of interest.
Figures




References
-
- Bolino A, D’Antonio M (2023) Recent advances in the treatment of Charcot-Marie-tooth neuropathies. J Peripher Nerv Syst 28:134–149. 10.1111/jns.12539 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

