Safety and tolerability of a Muse cell-based product in neonatal hypoxic-ischemic encephalopathy with therapeutic hypothermia (SHIELD trial)
- PMID: 39401019
- PMCID: PMC11555474
- DOI: 10.1093/stcltm/szae071
Safety and tolerability of a Muse cell-based product in neonatal hypoxic-ischemic encephalopathy with therapeutic hypothermia (SHIELD trial)
Abstract
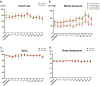
Hypoxic-ischemic encephalopathy (HIE), associated with high mortality and neurological sequelae, lacks established treatment except therapeutic hypothermia. Clinical-grade multilineage-differentiating stress-enduring (Muse) cells (CL2020) demonstrated safety and efficacy in nonclinical HIE rat models, thereby leading to an investigator-initiated clinical trial to evaluate CL2020 safety and tolerability in neonatal HIE as a single-center open-label dose-escalation study with 9 neonates with moderate-to-severe HIE who received therapeutic hypothermia. Each patient received a single intravenous injection of CL2020 cells between 5 and 14 days of age. The low-dose (3 patients) and high-dose (6 patients) groups received 1.5 × 106 and 1.5 × 107 cells/dose, respectively. The occurrence of any adverse event within 12 weeks following CL2020 administration was the primary endpoint of this trial. No significant changes in physiological signs including heart rate, blood pressure, and oxygen saturation were observed during or after administration. The only adverse event that may be related to cell administration was a mild γ-glutamyltransferase level elevation in one neonate, which spontaneously resolved without any treatment. All patients enrolled in the trial survived, and normal developmental quotients (≥ 85) in all 3 domains of the Kyoto Scale of Psychological Development 2001 were observed in 67% of the patients in this trial. CL2020 administration was demonstrated to be safe and tolerable for neonates with HIE. Considering the small number of patients, a randomized controlled confirmatory study is warranted to verify these preliminary findings and evaluate the efficacy of this therapy.
Keywords: Muse; cerebral palsy; hypothermia; hypoxic–ischemic encephalopathy; mesenchymal stem cell; neonates.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
Y.S. and S.Sh. had collaborative projects with research funding from LSII for perinatal diseases. S.Sh. and A.H. received fees based on a consultation contract from LSII. Y.S., S.S., T.S., and M.H. have a patent for the application of Muse cells in the treatment of HIE and other indications. LSII provided CL2020 for this clinical trial free of charge. The LSII was not involved in the design, conduct, or analysis of the results.
Figures


References
-
- Centers for Disease Control and Prevention (CDC). Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment--United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53(3):57-59. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

