A comparative study of the cryo-EM structures of Saccharomyces cerevisiae and human anaphase-promoting complex/cyclosome (APC/C)
- PMID: 39401078
- PMCID: PMC11473103
- DOI: 10.7554/eLife.100821
A comparative study of the cryo-EM structures of Saccharomyces cerevisiae and human anaphase-promoting complex/cyclosome (APC/C)
Abstract
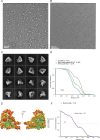
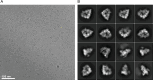
The anaphase-promoting complex/cyclosome (APC/C) is a large multi-subunit E3 ubiquitin ligase that controls progression through the cell cycle by orchestrating the timely proteolysis of mitotic cyclins and other cell cycle regulatory proteins. Although structures of multiple human APC/C complexes have been extensively studied over the past decade, the Saccharomyces cerevisiae APC/C has been less extensively investigated. Here, we describe medium resolution structures of three S. cerevisiae APC/C complexes: unphosphorylated apo-APC/C and the ternary APC/CCDH1-substrate complex, and phosphorylated apo-APC/C. Whereas the overall architectures of human and S. cerevisiae APC/C are conserved, as well as the mechanism of CDH1 inhibition by CDK-phosphorylation, specific variations exist, including striking differences in the mechanism of coactivator-mediated stimulation of E2 binding, and the activation of APC/CCDC20 by phosphorylation. In contrast to human APC/C in which coactivator induces a conformational change of the catalytic module APC2:APC11 to allow E2 binding, in S. cerevisiae apo-APC/C the catalytic module is already positioned to bind E2. Furthermore, we find no evidence of a phospho-regulatable auto-inhibitory segment of APC1, that in the unphosphorylated human APC/C, sterically blocks the CDC20C-box binding site of APC8. Thus, although the functions of APC/C are conserved from S. cerevisiae to humans, molecular details relating to their regulatory mechanisms differ.
Keywords: APC/C; S. cerevisiae; cell cycle; chromosomes; gene expression; mitosis; ubiquitination.
© 2024, Vazquez-Fernandez et al.
Conflict of interest statement
EV, JY, ZZ, AA, PE, DB No competing interests declared
Figures















Update of
References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallographica. Section D, Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

