Preparation and Characterization of Photo-Cross-Linkable Methacrylated Silk Fibroin and Methacrylated Hyaluronic Acid Composite Hydrogels
- PMID: 39401165
- PMCID: PMC11558566
- DOI: 10.1021/acs.biomac.4c00319
Preparation and Characterization of Photo-Cross-Linkable Methacrylated Silk Fibroin and Methacrylated Hyaluronic Acid Composite Hydrogels
Abstract
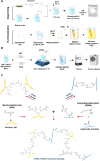
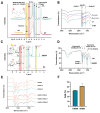
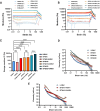
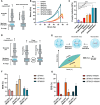
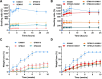
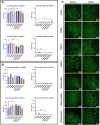
Composite biomaterials with excellent biocompatibility and biodegradability are crucial in tissue engineering. In this work, a composite protein and polysaccharide photo-cross-linkable hydrogel was prepared using silk fibroin methacrylate (SFMA) and hyaluronic acid methacrylate (HAMA). SFMA was obtained by the methacrylation of degummed SF with glycidyl methacrylate (GMA), while HA was methacrylated by 2-aminoethyl methacrylate hydrochloride (AEMA). We investigated the effect of the addition of 1 wt % HAMA to 5, 10, and 20 wt % SFMA, which resulted in an increase in both static and cycling mechanical strengths. All composite hydrogels gelled under UV light in <30 s, allowing for rapid stabilization and stiffness increases. The biocompatibility of the hydrogels was confirmed by direct and indirect contact methods and by evaluation against the NIH3T3 and MC3T3 cell lines with a live-dead assay by confocal imaging. The range of obtained mechanical properties from developed composite and UV-cross-linkable hydrogels sets the basis as possible future biomaterials for various biomedical applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Salma-Ancane K.; Sceglovs A.; Tracuma E.; Wychowaniec J. K.; Aunina K.; Ramata-Stunda A.; Nikolajeva V.; Loca D. Effect of crosslinking strategy on the biological, antibacterial and physicochemical performance of hyaluronic acid and ε-polylysine based hydrogels. Int. J. Biol. Macromol. 2022, 208, 995–1008. 10.1016/j.ijbiomac.2022.03.207. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

