Gene therapy for fat-1 prevents obesity-induced metabolic dysfunction, cellular senescence, and osteoarthritis
- PMID: 39401356
- PMCID: PMC11513907
- DOI: 10.1073/pnas.2402954121
Gene therapy for fat-1 prevents obesity-induced metabolic dysfunction, cellular senescence, and osteoarthritis
Abstract
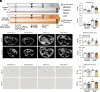
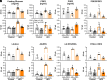
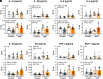
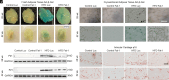
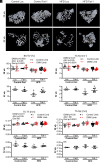
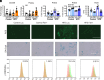
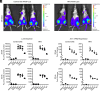
Obesity is one of the primary risk factors for osteoarthritis (OA), acting through cross talk among altered biomechanics, metabolism, adipokines, and dietary free fatty acid (FA) composition. Obesity and aging have been linked to cellular senescence in various tissues, resulting in increased local and systemic inflammation and immune dysfunction. We hypothesized that obesity and joint injury lead to cellular senescence that is typically associated with increased OA severity or with aging and that the ratio of omega-6 (ω-6) to omega-3 (ω-3) FAs regulates these pathologic effects. Mice were placed on an ω-6-rich high-fat diet or a lean control diet and underwent destabilization of the medial meniscus to induce OA. Obesity and joint injury significantly increased cellular senescence in subcutaneous and visceral fat as well as joint tissues such as synovium and cartilage. Using adeno-associated virus (AAV) gene therapy for fat-1, a fatty acid desaturase that converts ω-6 to ω-3 FAs, decreasing the serum ω-6:ω-3 FA ratio had a strong senomorphic and therapeutic effect, mitigating metabolic dysfunction, cellular senescence, and joint degeneration. In vitro coculture of bone marrow-derived macrophages and chondrocytes from control and AAV8-fat1-treated mice were used to examine the roles of various FA mediators in regulating chondrocyte senescence. Our results suggest that obesity and joint injury result in a premature "aging" of the joint as measured by senescence markers, and these changes can be ameliorated by altering FA composition using fat-1 gene therapy. These findings support the potential for fat-1 gene therapy to treat obesity- and/or injury-induced OA clinically.
Keywords: AAV; gene therapy; obesity; osteoarthritis; senescence.
Conflict of interest statement
Competing interests statement:F.G. is an employee and shareholder in Cytex Therapeutics, Inc. R.T., N.S.H., C.-L.W., and F.G. have received royalties from Agathos Biologics.
Figures


References
-
- Hales C. M., Carroll M. D., Fryar C. D., Ogden C. L., Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 360, 1–8 (2020). - PubMed
-
- Felson D. T., Anderson J. J., Naimark A., Walker A. M., Meenan R. F., Obesity and knee osteoarthritis. The Framingham Study. Ann. Intern. Med. 109, 18–24 (1988). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

