Radiomultiomics: quantitative CT clusters of severe asthma associated with multiomics
- PMID: 39401856
- PMCID: PMC11579543
- DOI: 10.1183/13993003.00207-2024
Radiomultiomics: quantitative CT clusters of severe asthma associated with multiomics
Abstract
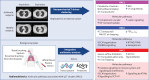
Background: Lung quantitative computed tomography (qCT) severe asthma clusters have been reported, but their replication and underlying disease mechanisms are unknown. We identified and replicated qCT clusters of severe asthma in two independent asthma cohorts and determined their association with molecular pathways, using radiomultiomics, integrating qCT, multiomics and machine learning/artificial intelligence.
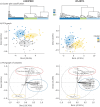
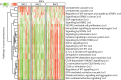
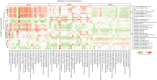
Methods: We used consensus clustering on qCT measurements of airway and lung CT scans, performed in 105 severe asthmatic adults from the U-BIOPRED cohort. The same qCT measurements were used to replicate qCT clusters in a subsample of the ATLANTIS asthma cohort (n=97). We performed integrated enrichment analysis using blood, sputum, bronchial biopsies, bronchial brushings and nasal brushings transcriptomics and blood and sputum proteomics to characterise radiomultiomic-associated clusters (RACs).
Results: qCT clusters and clinical features in U-BIOPRED were replicated in the matched ATLANTIS cohort. In the U-BIOPRED cohort, RAC1 (n=30) was predominantly female with elevated body mass index, mild airflow limitation, decreased CT lung volume and increased lung density and upregulation of the complement pathway. RAC2 (n=34) subjects had airway wall thickness and a mild degree of airflow limitation, with upregulation of proliferative pathways including neurotrophic receptor tyrosine kinase 2/tyrosine kinase receptor B, and downregulation of semaphorin pathways. RAC3 (n=41) showed increased lung attenuation area and air trapping, severe airflow limitation, hyperinflation, and upregulation of cytokine signalling and signalling by interleukin pathways, and matrix metallopeptidase 1, 2 and 9.
Conclusions: U-BIOPRED severe asthma qCT clusters were replicated in a matched independent asthmatic cohort and associated with specific molecular pathways. Radiomultiomics might represent a novel strategy to identify new molecular pathways in asthma pathobiology.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: K.F. Chung reports grants from the MRC, EPSRC, GSK, Merck and NIEHS, payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK, Novartis and AstraZeneca, and participation on a data safety monitoring board or advisory board with GSK, AstraZeneca, Novartis, Roche, Merck, Trevi, Rickett-Beckinson, Nocion, Shionogi and Clean Breathing Institute supported by Haleon. C. Auffray reports support for the present study from the Innovative Medicines Initiative. Y. Badi reports support for the present study from the Innovative Medicines Initiative. P. Bakke reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GSK and Sanofi. C. Brightling reports support for the present study from the ATLANTIS study (Chiesi grant) and Leicester NIHR BRC, grants from 4D Pharma, Areteia, AstraZeneca, Chiesi, Genentech, GSK, Mologic, Novartis, Regeneron Pharmaceuticals, Roche and Sanofi, and consultancy fees from 4D Pharma, Areteia, AstraZeneca, Chiesi, Genentech, GSK, Mologic, Novartis, Regeneron Pharmaceuticals, Roche and Sanofi. P. Chanez reports grants from ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Menarini, Novartis and Sanofi Aventis, consultancy fees from ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Menarini, Novartis and Sanofi Aventis, payment or honoraria for lectures, presentations, manuscript writing or educational events from ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Menarini, Novartis and Sanofi Aventis, and support for attending meetings from ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Menarini, Novartis and Sanofi Aventis. B. De Meulder reports support for the present study from the Innovative Medicines Initiative. R. Djukanovic reports consultancy fees from Synairgen plc, GSK, ZenasBio and Celltrion, leadership role as Chair of the European Respiratory Society's clinical research collaboration on severe asthma (SHARP), and stock (or stock options) with Synairgen plc. L. Fabbri reports consultancy fees from Chiesi Farmaceutici and AstraZeneca, payment or honoraria for lectures, presentations, manuscript writing or educational events from Chiesi Farmaceutici, AstraZeneca, GSK, Alfasigma and Novartis, support for attending meetings from Chiesi Farmaceutici, GSK and Novartis, and participation on a data safety monitoring board or advisory board with Novartis and Chiesi. S.J. Fowler reports grants from the NIHR, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Boehringer Ingelheim. P. Howarth reports employment with GSK. J. Kolmert reports consultancy fees from Gesynta Pharma AB and Lipum AB. M. Kraft reports grants from the NIH, American Lung Association, Synairgen, Janssen, AstraZeneca and Sanofi, consultancy fees from AstraZeneca, Sanofi, Chiesi, GSK, Kinaset and Genentech, payment or honoraria for lectures, presentations, manuscript writing or educational events from Chiesi, support for attending meetings from the European Respiratory Society, one patent issued and tiled filed to development of therapeutics for inflammatory lung disease as CoFounder and Chief Medical Officer, RaeSedo, Inc., participation on a data safety monitoring board or advisory board with ALung, leadership role with the National Heart, Lung and Blood Advisory Council, stock (or stock options) with RaeSedo, Inc. (equity ownership), and the following financial (or non-financial) interests: Section Editor of UpToDate. A.H. Maitland-van der Zee reports grants from Health Holland, GSK, Boehringer Ingelheim and Vertex, consultancy fees from Boehringer Ingelheim and AstraZeneca, payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK, and participation on a data safety monitoring board or advisory board for a study on BPD in neonates. A. Papi reports grants from Chiesi, AstraZeneca, GSK and Sanofi, consultancy fees from Chiesi, AstraZeneca, GSK, Novartis, Sanofi, Iqvia, Avillion, Elpen Pharmaceuticals, Moderna and Roche, payment or honoraria for lectures, presentations, manuscript writing or educational events from Chiesi, AstraZeneca, GSK, Menarini, Zambon, Mundipharma, Sanofi, Edmond Pharma, Iqvia, Avillion, Sanofi and Regeneron, participation on a data safety monitoring board or advisory board with Chiesi, AstraZeneca, GSK, Novartis, Sanofi, Iqvia, Avillion, Elpen Pharmaceuticals and Moderna, receipt of equipment, materials, drugs, medical writing, gifts or other services from Consorzio Futuro in Ricerca. K. Rabe reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, Novartis, Sanofi/Regeneron, GSK, Berlin Chemie and Roche Pharma, participation on a data safety monitoring board or advisory board with AstraZeneca, Boehringer Ingelheim, Sanofi/Regeneron and CSL Behring, and leadership roles with the German Center for Lung Research (DZL), German Chest Society (DGP) and American Thoracic Society. D.E. Shaw reports consultancy fees from GSK, AstraZeneca and Novartis, payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK and AstraZeneca, and support for attending meetings from GSK. D. Singh reports consultancy fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, EpiEndo, Genentech, GSK, Glenmark, Gossamer Bio, Kinaset Therapeutics, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Synairgen, Teva, Theravance, Biopharma and Verona Pharma. M. van Den Berge reports grants from GSK, Roche, Genentech and Novartis. Y. Guo reports support for the present study from the Innovative Medicines Initiative. S. Wagers reports consultancy fees from King's College Hospital NHS Foundation Trust, Academic Medical Research, AMC Medical Research BV, Asthma UK, Athens Medical School, Boehringer Ingelheim International GmbH, CHU de Toulouse, CIRO, DS Biologicals Ltd, École Polytechnique Fédérale De Lausanne, European Respiratory Society, FISEVI, Fluidic Analytics Ltd, Fraunhofer IGB, Fraunhofer ITEM, GSK Research & Development Ltd, Holland & Knight, Karolinska Institutet Fakturor, KU Leuven, Longfonds, National Heart and Lung Institute, Novartis Pharma AG, Owlstone Medical Limited, PExA AB, UCB Biopharma SPRL, Umeå University, University Hospital Southampton NHS Foundation Trust, Università Campus Bio-Medico di Roma, Universita Cattolica Del Sacro Cuore, Universität Ulm, University of Bern, University of Edinburgh, University of Hull, University of Leicester, University of Loughborough, University of Luxembourg, University of Manchester, University of Nottingham, Vlaams Brabant, Dienst Europa, Imperial College London, Boehringer Ingelheim, Breathomix, Gossamer Bio, AstraZeneca, CIBER, OncoRadiomics, University of Leiden, University of Wurzburg, Chiesi Pharmaceutical, University of Liege, Teva Pharmaceuticals, Sanofi, Pulmonary Fibrosis Foundation and Three Lakes Foundation. P.J. Sterk reports consultancy fees from SME Breathomix, and stock (or stock options) with SME Breathomix. S-E. Dahlen reports grants from AstraZeneca, GSK and Sanofi, consultancy fees from AstraZeneca, Cayman Chemicals, GSK and Regeneron, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GSK and Sanofi. I.M. Adcock reports support for the present study from the Innovative Medicines Initiative, grants from GSK, MRC, EPSRC and Sanofi, consultancy fees from GSK, Sanofi, Chiesi and Kinaset, payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Sanofi, Eurodrug and Sunovion, and support for attending meetings from AstraZeneca. S. Siddiqui consultancy fees from AstraZeneca, GSK, CSL Behring, Areteia Therapeutics, ERT Medical, Chiesi and Roche, and payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK, Chiesi and AstraZeneca. P. Montuschi reports support for the present study from the Innovative Medicines Initiative. The remaining authors have no potential conflicts of interest to disclose.
Figures


Comment in
-
Radiomultiomics: pioneering precision medicine for severe asthma.Eur Respir J. 2024 Nov 21;64(5):2401639. doi: 10.1183/13993003.01639-2024. Print 2024 Nov. Eur Respir J. 2024. PMID: 39572207 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
