Comparison of oronasal and nasal masks in home mechanical ventilation: an observational cohort and bench study
- PMID: 39401860
- PMCID: PMC11694558
- DOI: 10.1183/13993003.02010-2023
Comparison of oronasal and nasal masks in home mechanical ventilation: an observational cohort and bench study
Abstract
Background: In patients with chronic respiratory failure, home non-invasive ventilation (NIV) is delivered through oronasal or nasal masks. Masks are a cornerstone for NIV success but can be associated with side-effects. However, the type, frequency and consequences of these side-effects are unknown. Here, we aimed to study the prevalence, nature and impact of mask-related adverse events in a cohort of stable patients. We then investigated differences between oronasal and nasal masks both in our cohort and in a bench study.
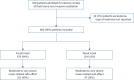
Methods: This was a prospective observational cohort including patients established on long-term NIV admitted for their elective review. Data regarding mask-related side-effects were assessed using a structured questionnaire. Our bench study was performed using a three-dimensional printed head connected to an artificial lung.
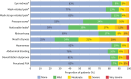
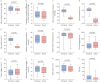
Results: 800 patients were included, of whom 84% had an oronasal mask. Moderate to very severe mask-related side-effects occurred in 47% of patients and severe to very severe side-effects occurred in 18% of patients. Side-effects were associated with poorer daytime arterial partial pressure of carbon dioxide (p=0.005), poorer subjective sleep quality (p=0.003) and poorer quality of life (p<0.001). Mask-related side-effects were more frequently reported with the use of oronasal masks compared to nasal masks (p=0.023). Our bench study showed that nasal masks were more stable than oronasal masks (p<0.001).
Conclusions: Mask-related side-effects are frequent and associated with poorer outcomes. Our data suggest that nasal masks may have a better tolerance profile and should be used as a first-line interface.
Copyright ©The authors 2025.
Conflict of interest statement
Conflicts of interest: E. Fresnel reports support for the present study from ADIR Association, grants from ADIR Association and Adène Groupe, a leadership role as CEO of Kernel Biomedical, and is employed as a research engineer by Kernel Biomedical. C. Caillard reports support for the present study from Asten Santé and Kernel Biomedical, and support for attending meetings from Asten Santé and SOS Oxygène. M. Lebret reports grants from ADIR Association and Aden scientific fund, support for attending meetings from Air Liquide Medical Systems and Kernel Biomedical, and is a part-time employee of Air Liquide Medical Systems (medical affairs department) and part-time employee of Kernel Biomedical (research engineer). L. Razakamanantsoa reports support for attending meetings from Asten Santé and ASV Santé. A. Kerfourn reports support for the present study from ADIR Association, grants from ADIR Association and Adène Groupe, leadership role as CEO of Kernel Biomedical, and is employed as a research engineer by Kernel Biomedical. J. Dupuis is an employee of Asten Santé. J-F. Muir receives a salary from ADIR Association as President. E. Lhuillier reports support for attending meetings from Asten Santé. T. Similowski reports consultancy fees from AstraZeneca, Chiesi, KPL consulting, Lungpacer Inc. and OSO-AI, payment or honoraria for lectures, presentations, manuscript writing or educational events from Chiesi and Vitalaire, patents planned, issued or pending (WO2008006963A3, WO2012004534A1, WO2013164462A1), and stock (or stock options) with Austral Dx and Hephai. M. Patout reports grants from Fisher & Paykel, ResMed and Asten Santé, consultancy fees from Philips Respironics, Air Liquide Medical, ResMed, Asten Santé and GSK, payment or honoraria for lectures, presentations, manuscript writing or educational events from Philips Respironics, Asten Santé, ResMed, Air Liquide Medical, SOS Oxygène, Antadir, Chiesi, Jazz Pharmaceutical, Loewenstein, Fisher & Paykel, Bastide Medical, Orkyn and Elivie, support for attending meetings from Asten Santé and Vitalaire, participation on a data safety monitoring board or advisory board with ResMed, Philips Respironics and Asten Santé, stock (or stock options) with Kernel Biomedical, and receipt of equipment, materials, drugs, medical writing, gifts or other services from Philips Respironics, ResMed and Fisher & Paykel. The remaining authors have no potential conflicts of interest to disclose.
Figures



Comment in
-
The interface in home non-invasive ventilation: is the nasal mask better?Eur Respir J. 2025 Jan 2;65(1):2402007. doi: 10.1183/13993003.02007-2024. Print 2025 Jan. Eur Respir J. 2025. PMID: 39746772 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
